A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Herbivore-induced Blueberry Volatiles and Intra-plant Signaling
In This Article
Summary
A push-pull method for collecting plant volatiles is described. The method allows for a comparison of volatiles induced by herbivore feeding, exogenous methyl jasmonate, and mechanical damage. This technique is also used to investigate the volatile response of undamaged branches to exposure to volatiles from herbivore-damaged branches within blueberry plants.
Abstract
Herbivore-induced plant volatiles (HIPVs) are commonly emitted from plants after herbivore attack1,2. These HIPVs are mainly regulated by the defensive plant hormone jasmonic acid (JA) and its volatile derivative methyl jasmonate (MeJA)3,4,5. Over the past 3 decades researchers have documented that HIPVs can repel or attract herbivores, attract the natural enemies of herbivores, and in some cases they can induce or prime plant defenses prior to herbivore attack. In a recent paper6, I reported that feeding by gypsy moth caterpillars, exogenous MeJA application, and mechanical damage induce the emissions of volatiles from blueberry plants, albeit differently. In addition, blueberry branches respond to HIPVs emitted from neighboring branches of the same plant by increasing the levels of JA and resistance to herbivores (i.e., direct plant defenses), and by priming volatile emissions (i.e., indirect plant defenses). Similar findings have been reported recently for sagebrush7, poplar8, and lima beans9..
Here, I describe a push-pull method for collecting blueberry volatiles induced by herbivore (gypsy moth) feeding, exogenous MeJA application, and mechanical damage. The volatile collection unit consists of a 4 L volatile collection chamber, a 2-piece guillotine, an air delivery system that purifies incoming air, and a vacuum system connected to a trap filled with Super-Q adsorbent to collect volatiles5,6,10. Volatiles collected in Super-Q traps are eluted with dichloromethane and then separated and quantified using Gas Chromatography (GC). This volatile collection method was used n my study6 to investigate the volatile response of undamaged branches to exposure to volatiles from herbivore-damaged branches within blueberry plants. These methods are described here. Briefly, undamaged blueberry branches are exposed to HIPVs from neighboring branches within the same plant. Using the same techniques described above, volatiles emitted from branches after exposure to HIPVs are collected and analyzed.
Protocol
1. Local induction of volatiles: herbivore damage
- Two branches of blueberry plants are bagged with a spun polyester sleeve.
- Six gypsy moth caterpillars (2nd-3rd instars) are placed inside the bags and allowed to feed on plants for 2 days prior to volatile collection. Control plants do not receive any caterpillars.
- Volatile emissions are collected as described below (protocol #7) on day 3 (Figure 1). Polyester sleeves remain on plants to prevent insects from escaping.
2. Local induction of volatiles: mechanical damage
- Mechanical damage to plants is inflicted by punching holes to mimic the amount of leaf area removed by gypsy moth.
- Five leaves per plant are injured with two 7-mm holes located at the base and upper portion of leaves at the end of days 1 and 2. The control treatment does not receive mechanical damage.
- Volatile emissions are collected as described below on day 3.
3. Local induction of volatiles: MeJA
- Blueberry plants are treated with 10 mL of either a 1 or 1.5 mM solution of MeJA in a 0.1% Tween-20 solution.
- MeJA is applied using a 2-oz spray bottle. Control plants are sprayed with 10 mL of a 0.1% Tween-20 solution.
- Plants are treated at 16:00 hr, and kept in the greenhouse for 15 hr inside a 17-cm diameter and 35-cm high Plexiglas chamber prior to volatile collections.
- Volatile emissions are collected as described below.
4. Systemic induction of volatiles: internal signaling
- A lower branch of a blueberry plant is bagged with a spun polyester sleeve and damaged by placing six (2nd-3rd instars) gypsy moth caterpillars inside the bag.
- The damaged branch remains outside the volatile collection chamber, while the branches above the damaged branch are placed inside the chamber (Figure 2).
- Caterpillars are allowed to feed on the bottom branch for 2 days.
- Starting on day 3, volatiles are collected from the undamaged portion of damaged plants.
- Volatiles are collected for 7 consecutive days. Control plants are treated in a similar manner but did not receive herbivore damage.
5. Vascular connectivity
- This study determined the degree of vascular connectivity among different branches within a blueberry plant.
- The end portion of a lower branch from a plant is cut to support a floral water pick containing 6 mL solution of a Rhodamine-B (Sigma-Aldrich) dye (0.25% w/v), as described in Orians et al.11
- Movement of the dye through the plant is monitored daily for 7 days.
- After 7 days, the amount of red staining is visually assessed from different positions of the plant.
6. Exposure to HIPVs: external signaling
- Branches within a plant are either exposed to HIPVs from an adjacent branch or received no exposure to HIPVs.
- To expose branches to HIPVs, one lower branch is bagged with a polyester sleeve and six (2nd-3rd instars) gypsy moth caterpillars are placed inside bags (Figure 3).
- The plant is caged inside a Plexiglas chamber similar to those described above. Insects are allowed to feed on plants for 2 days, such that adjacent branches are exposed to volatiles emitted from the induced branch.
- After exposure (day 3), exposed branches are placed inside the volatile collection chamber, leaving the insect-injured branch outside (as seen in Figure 2).
- Volatiles from HIPV-exposed branches are collected as described below, and the amount of leaf area consumed from the lower branch are measured using Scion Image Software.
7. Exposure to HIPVs: priming
- To determine whether plants are "primed" after HIPV exposure, plants are treated as in protocol #5.
- Half of the plants are exposed to HIPVs from an induced adjacent branch; while the other half is exposed to undamaged branches.
- On day 3, four early 2nd-instar gypsy moth caterpillars are placed on each plant and volatiles are collected as described below (Figure 4).
- After volatile collections, leaves initially damaged by gypsy moth caterpillars, as well as those that are damaged on day 3, are excised and amounts of leaf area consumed are measured.
8. Collection of volatiles
- HIPV emissions are sampled in the greenhouse using a push-pull system. The above ground portion of potted plants (stem, branches, and leaves), including caterpillars in the herbivore damage treatment, are placed inside a 4 L volatile collection chamber. A 2-piece guillotine supports the base of plants. Purified air enters the top of each chamber at a rate of 2 L/min.
- Volatiles are collected in traps filled with 30 mg of Alltech Super-Q adsorbent by pulling air from the chambers at a rate of 1 L/min.
- The volatile collection apparatus consists of four chambers, allowing for simultaneous collections of volatiles from four different plants (Figure 1).
- Collections are conducted during daytime from 09:00 to 17:00 h.
- After collection, all leaves from plants are harvested, oven dried at 60 °C, and weighed, and chambers are rinsed with tap water and 70% ethanol.
9. Analysis of volatiles
- The collected volatiles from Super-Q traps are eluted with dichloromethane (150 μl) and 400 ng of n-octane is added as internal standard.
- Separation and quantification of compounds is done on a Hewlett Packard 6890 Series Gas Chromatograph (GC) (Figure 5), equipped with a flame ionization detector (FID) and an Agilent HP-1 column (10 m x 0.53 mm x 2.65 μm), and using He as carrier gas (constant flow = 5 ml/min, velocity = 39 cm/sec). The temperature program started at 40 °C kept for 1 min, increased at 14 °C/min to 180 °C (2 min), then at 40 °C/min to 200 °C, and kept at 200 °C for 2 min.
- Tentative identification of compounds is carried out on a Varian 3400 GC coupled to a Finnigan MAT 8230 mass spectrometer (MS), equipped with a Supelco MDN-5S column (30 m x 0.32 mm x 0.25 μm), and with He as the carrier gas. The MS is operated in electron ionization (EI) and total ion chromatogram (TIC) mode at 250 °C (source temperature). The program started at 35 °C (1 min), increased at 4 °C/min to 170 °C, then at 15 °C/min to 280 °C.
- Compounds are tentatively identified by comparison of spectral data with those from NIST library and by GC retention index, and by comparing their retention times with those of commercially available compounds.
10. Representative Results:
Twenty-two volatiles were identified from blueberry leaves (Figure 6). Figure 7 shows a representative chromatograph of undamaged blueberry leaves and leaves damaged by feeding by gypsy moth. Mechanical damage and feeding by gypsy moth caterpillars increased volatiles emissions locally from blueberry leaves compared to controls (Figure 8). Compared to caterpillar feeding, the MeJA treatment induced 11 out of the 17 compounds induced by gypsy moth (Figure 9). There was, however, no evidence of systemic induction of volatiles from undamaged leaves of gypsy moth-damaged plants seven days after initial feeding damage (i.e., lack of internal signaling) (Figure 10). In addition, after one week, very slow movement of the red dye was observed among branches of blueberry plants (Figure 11). There was high vascular connectivity among leaves within a single branch. However, there was intermediate-to-low connectivity between two branches vertically aligned within a shoot, and low connectivity between two branches on opposite sides of a shoot.
There was no difference between amounts of volatiles emitted from branches exposed to HIPVs versus those not exposed to HIPVs (Figure 12). However, HIPVs act as external defensive signals in blueberries. Gypsy moth caterpillars fed on leaves previously exposed to HIPVs consumed 71% less leaf material than did those fed on unexposed control leaves (Figure 13). Furthermore, amounts of volatiles emitted per amount of leaf area consumed in HIPV-exposed branches were 4-fold higher compared to unexposed branches (Figure 14), indicating that leaves from HIPV-exposed branches were more responsive to herbivory (i.e., they were primed).
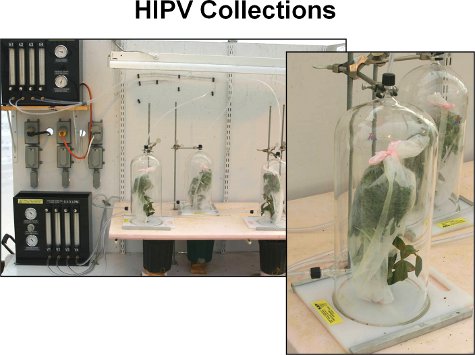
Figure 1. A push-pull system is used to collect volatiles from blueberry plants. Plants are placed inside glass chambers and clean air is passed over them. A filter containing an adsorbent material was attached to the side of each chamber to trap volatiles emitted from the plant. A vacuum is used to pull air from the inside of the chamber through the filter.
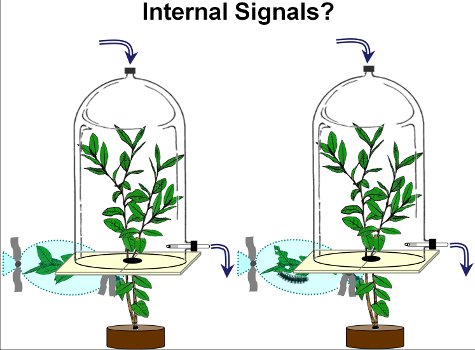
Figure 2. To study the systemic volatile response from blueberry plants, lower blueberry branches were either damaged by gypsy moth caterpillars (right chamber) or left undamaged (left chamber). After 2 days (on day 3), volatiles from undamaged upper branches from damaged and undamaged plants were collected.

Figure 3. To test whether leaves from undamaged branches respond to HIPVs from damaged branches, I bagged one of the branches in each of blueberry plants. I then placed gypsy moth caterpillars inside the bags of half of the plants. The bags I used allow movement of volatiles from inside to the outside of bags. Plants were placed inside Plexiglas chambers to expose undamaged branches to HIPVs. Volatiles were then collected from exposed branches.

Figure 4. To test whether leaves from undamaged branches are "primed" after exposure to HIPVs, experiments were repeated as described in Figure 3 but gypsy moth caterpillars were placed on HIPV-exposed and unexposed branches inside each volatile collection chamber.

Figure 5. After volatile collections, samples are injected onto a gas chromatograph (GC) to identify and quantify the volatiles emitted from blueberry plants.
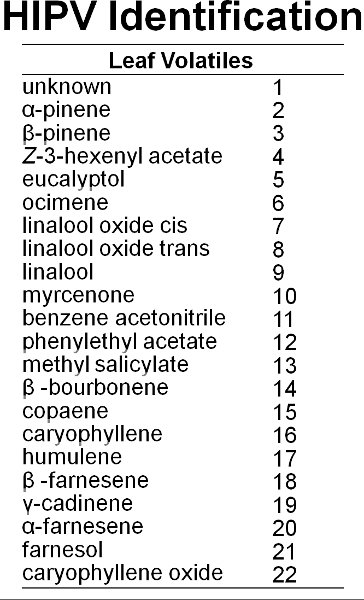
Figure 6. At least 22 compounds are emitted from blueberry leaves.

Figure 7. Typical chromatographs from undamaged blueberry leaves and leaves damaged by gypsy moth caterpillars. Volatiles are emitted at very low amounts from undamaged blueberry leaves. However, when leaves are damaged by gypsy moth caterpillars, emission of volatiles increased dramatically.
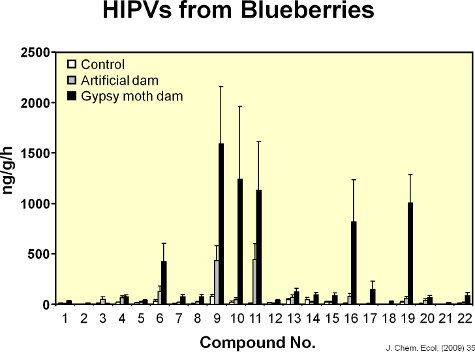
Figure 8. The graph shows the amounts of each of 22 volatiles emitted from undamaged leaves, mechanically-damaged leaves, and leaves damaged by gypsy moth caterpillars. Artificial damage to mimic the amount of leaf area removed by caterpillars increased volatile emission from blueberry leaves but the response was different from the volatile response of leaves to gypsy moth feeding.

Figure 9. Tested whether the JA pathway regulates volatile emission in blueberry leaves. Plants were sprayed with different amounts of MeJA. I found that increasing concentrations of exogenously-applied MeJA increased emissions of volatiles from blueberry leaves.

Figure 10. The graph shows the total amounts of volatiles emitted from control blueberry branches and from undamaged branches from gypsy moth damaged plants (systemic response). Volatiles were collected for a total of 7 consecutive days. I found no systemic induction of volatiles even 7 days after initial damage to lower branches of plants.
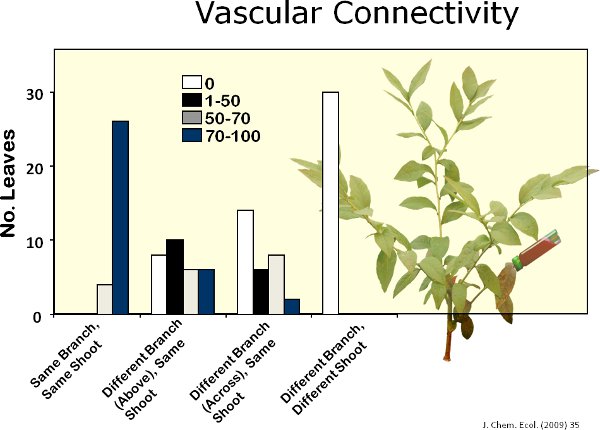
Figure 11. I used rhodamine-B (red dye) to determine the degree of vascular connectivity among branches within blueberry plants. I found that approx. 80%, 20%, 5% and 0% of leaves from branches containing the dye, branches directly above the branch containing the dye, branches across the branch containing dye, and branches located in a different shoot within a blueberry plant, respectively, were fully stained with the dye.

Figure 12. The graph shows the amount of volatiles emitted from branches exposed to HIPVs and unexposed branches. I found that exposure to HIPVs did not affect volatile emissions in neighboring undamaged blueberry branches.

Figure 13. The graph shows the amount of feeding by gypsy moth caterpillars on blueberry branches exposed to HIPVs and unexposed branches. Caterpillars on HIPV-exposed branches consumed less amount of foliage compared with those on unexposed branches.

Figure 14. When I calculated the emission rate per area consumed, I found that HIPV-exposed branches had increased emission rates of volatiles compared with unexposed branches, indicating that exposure to HIPVs primed leaves in blueberry branches for an increased volatile response.
Discussion
The push-pull volatile collection apparatus described here represents a standard method for headspace collections of plant volatiles. This apparatus was used to determine the volatile response of blueberry leaves to herbivory by gypsy moth caterpillars and also allowed me to provide new evidence for the role of HIPVs in intra-plant signaling.
The results presented here show that caterpillar feeding, exogenously-applied MeJA , and mechanical wounding increased volatile emissions at the site of...
Disclosures
I have nothing to disclose
Acknowledgements
The author thanks Robert Holdcraft for technical assistance. This study was funded in part by a USDA CSREES Special Grant (2009-34155-19957) and hatch funds (NJ08192).
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| Volatile collection chambers | Analytical Research Systems, Inc. | VCC-G6X12DT-1P | Gainesville, FL |
| Air compressor, 20 gal, oil free, 2 hp | Westward | 3JR71 | Sold by Grainger, Inc. |
| Air delivery system | Analytical Research Systems, Inc. | VCS-ADS-4AFM4C | Gainesville, FL |
| Air collection system | Analytical Research Systems, Inc. | VCS-MVCS-4CX1P | Gainesville, FL |
| Vacuum pump 100-150V, ¼ hp | Gast Manufacturing, Inc. | 4F740 | Sold by Grainger, Inc. |
| Methyl jasmonate | Sigma-Aldrich | J2500 | St. Louis, MO |
| Tween-20 | Sigma-Aldrich | 93773 | St. Louis, MO |
| Rhodamine-B | Sigma-Aldrich | St. Louis, MO | |
| Plastic spray bottles, 2 oz | Setco Inc. | Cranbury, NJ | |
| Spun polyester sleeves | Rockingham Opportunities Corp. | Reidsville, NC | |
| Super-Q volatile collection traps | Analytical Research Systems, Inc. | VCT-1/4X3-SPQ | Gainesville, FL |
| Scion Image Software | Scion Corporation | Frederick, MD | |
| Dichloromethane | Sigma-Aldrich | 270997 | St. Louis, MO |
| Gas chromatograph HP 6890 | Hewlett Packard | ||
| Gas chromatograph Varian 3400 | Varian | ||
| n-octane | Sigma-Aldrich | 296988 | St. Louis, MO |
| Mass spectrometer MAT 8230 | Finnigan | San Jose, CA | |
| HP-1 GC column | Agilent Technologies | Palo Alto, CA | |
| MDN-5S GC column | Supelco, Inc. | Bellefonte, PA |
References
- Dicke, M., Van Loon, J. J. A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97, 237-237 (2000).
- Mumm, R., Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 88, 628-628 (2010).
- Hopke, J. Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a β-glucosidase and jasmonic acid. FEBS Lett. 352, 146-146 (1994).
- Rodriguez-Saona, C. Behavioral and electrophysiological responses of the emerald ash borer, Agrilus planipennis, to induced volatiles of Manchurian ash, Fraxinus mandshurica. Chemoecology. 16, 75-75 (2006).
- Rodriguez-Saona, C. Herbivore induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J. Chem. Ecol. 35, 163-163 (2009).
- Karban, R. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology. 87, 922-922 (2006).
- Frost, C. J. Within-plant signalling by volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 10, 490-490 (2007).
- Heil, M., Bueno Silva, J. C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. U.S.A. 104, 5467-5467 (2007).
- Turlings, T. C. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 250, 1251-1251 (1990).
- Makovic, I. Volatiles involved in the nonhost rejection of Fraxinus pennsylvanica by Lymantria dispar larvae. J. Agric. Food Chem. 44, 929-929 (1996).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.