A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Convenient and General Expression Platform for the Production of Secreted Proteins from Human Cells
* These authors contributed equally
In This Article
Summary
In the post-human genomics era, the availability of recombinant proteins in native conformations is crucial to structural, functional and therapeutic research and development. Here, we describe a test- and large-scale protein expression system in human embryonic kidney 293T cells that can be used to produce a variety of recombinant proteins.
Abstract
Recombinant protein expression in bacteria, typically E. coli, has been the most successful strategy for milligram quantity expression of proteins. However, prokaryotic hosts are often not as appropriate for expression of human, viral or eukaryotic proteins due to toxicity of the foreign macromolecule, differences in the protein folding machinery, or due to the lack of particular co- or post-translational modifications in bacteria. Expression systems based on yeast (P. pastoris or S. cerevisiae) 1,2, baculovirus-infected insect (S. frugiperda or T. ni) cells 3, and cell-free in vitro translation systems 2,4 have been successfully used to produce mammalian proteins. Intuitively, the best match is to use a mammalian host to ensure the production of recombinant proteins that contain the proper post-translational modifications. A number of mammalian cell lines (Human Embryonic Kidney (HEK) 293, CV-1 cells in Origin carrying the SV40 larget T-antigen (COS), Chinese Hamster Ovary (CHO), and others) have been successfully utilized to overexpress milligram quantities of a number of human proteins 5-9. However, the advantages of using mammalian cells are often countered by higher costs, requirement of specialized laboratory equipment, lower protein yields, and lengthy times to develop stable expression cell lines. Increasing yield and producing proteins faster, while keeping costs low, are major factors for many academic and commercial laboratories.
Here, we describe a time- and cost-efficient, two-part procedure for the expression of secreted human proteins from adherent HEK 293T cells. This system is capable of producing microgram to milligram quantities of functional protein for structural, biophysical and biochemical studies. The first part, multiple constructs of the gene of interest are produced in parallel and transiently transfected into adherent HEK 293T cells in small scale. The detection and analysis of recombinant protein secreted into the cell culture medium is performed by western blot analysis using commercially available antibodies directed against a vector-encoded protein purification tag. Subsequently, suitable constructs for large-scale protein production are transiently transfected using polyethyleneimine (PEI) in 10-layer cell factories. Proteins secreted into litre-volumes of conditioned medium are concentrated into manageable amounts using tangential flow filtration, followed by purification by anti-HA affinity chromatography. The utility of this platform is proven by its ability to express milligram quantities of cytokines, cytokine receptors, cell surface receptors, intrinsic restriction factors, and viral glycoproteins. This method was also successfully used in the structural determination of the trimeric ebolavirus glycoprotein 5,10.
In conclusion, this platform offers ease of use, speed and scalability while maximizing protein quality and functionality. Moreover, no additional equipment, other than a standard humidified CO2 incubator, is required. This procedure may be rapidly expanded to systems of greater complexity, such as co-expression of protein complexes, antigens and antibodies, production of virus-like particles for vaccines, or production of adenoviruses or lentiviruses for transduction of difficult cell lines.
Protocol
1. Preparation Work - Constructs and Cell Cultures
Before starting the protocol, the gene of interest should be codon-optimized for expression in mammalian cells, and cloned into an appropriate expression vector using standard molecular biology techniques. In order to ensure the highest chance for successful expression, multiple variants of the gene of interest should be generated. Many mammalian expression vectors are available commercially and have various purification tags (polyhistidine, hemagglutinin, streptavidin, HALO-Tag, glutathione S-transferase, among others). We prefer to use the pDISPLAY vector, which encodes for a strong human cytomegalovirus promoter, an Ig κ secretion signal, hemagglutinin purification tag, and has a C-terminal transmembrane anchor to target the protein through the secretory pathway for display on the plasma membrane. We usually insert a stop codon in front of the vector-encoded transmembrane anchor to allow the protein to be secreted into the conditioned medium.
Human embryonic kidney (HEK) 293T cells are widely available, and easily cultured and transfected. HEK 293T are routinely used for expression of mammalian proteins, but are considered biohazardous and should be handled at biosafety level 2. Please wear proper personal protective clothing; work should be performed in an approved biosafety cabinet using aseptic technique. All waste and surfaces should be disinfected according to institutional and governmental guidelines. It is recommended that cells be tested for mycoplasma contamination prior to use. Cells may be treated with ciprofloxacin (10 μg/ml) for ten days to eradicate any source of mycoplasma spp. contamination. General protocols to propagate HEK 293T cells are presented separately (Box 1).
Additional considerations for test- and large-scale protein expression are reviewed in 11-15.
2. Small-scale Test Expression
Once constructs have been designed and generated, small-scale test transfections may be performed using HEK 293T cells; a schematic summarizing the process is presented below (Fig. 1).
- Use T75 cm2 or T225 cm2 cell culture flasks (depending on the number of test expressions to be performed) to grow the HEK 293T cells and split the cells every 2-3 days when cells are 100% confluent (Box 1).
- Seed 2.5 x 105 HEK 293T cells per well in a 6-well plate and add 2 ml DMEM with 1X pen/strep and 10% (v/v) FBS, swirl the plate gently to ensure even cell dispersal in each well, and incubate overnight at 37 °C in a 5% CO2 humidified chamber.
- When HEK 293T cells reach 40% confluency, discard the media and add fresh 2 ml DMEM with 1X pen/strep and 10% (v/v) FBS to the wells. Perform transfection assays.
- Aliquot 90 μl serum-free DMEM into a sterile 1.5 ml microcentrifuge tube. Pipette 3 μl GeneJuice into the serum-free DMEM and gently mix the tube (finger vortex). Incubate for 5 minutes at room temperature.
- Add 1 μg of MiniPrep purified plasmid DNA (DNA stock= 100 ng/μl) into DMEM-GeneJuice mixture, finger vortex, and incubate for 15 minutes at room temperature.
- Pipette the transfection mixture dropwise onto HEK 293T cells, and swirl the 6-well plate gently to allow even distribution of the transfection mixture. Incubate the 6-well plate at 37 °C in a 5% CO2 humidified chamber.
- Add 1 ml fresh DMEM with 1X pen/strep and 10% (v/v) FBS to each well 24 hours post-transfection and incubate for another 48 hours (total 72 hours).
- Harvest 1 ml of supernatant from each well at three days post-transfection and microcentrifuge the samples at 16,000 g for 10 minutes at room temperature. Carry out western blot analysis as detailed in Box 2. Samples can be stored at 4 °C. The length of storage at 4 °C is protein dependent.
3. Large-scale Test Expression and Purification
Once a construct has been identified milligram quantity expression of recombinant protein is achieved by PEI transfection of adherent HEK 293T cells using 10-layer cell factories (Fig. 2; 6360 cm2 surface area). For more exploratory studies, smaller cell factories or T-flasks (Table 1) can be used.
- Purify 1 mg of DNA for transfection using a MaxiPrep plasmid purification kit. A 500 ml overnight culture of XL-1 Blue cells should produce at least 1 mg of pure DNA. Check purity of DNA by measuring A260/A280 ratio; should be above 1.8.
- Scale up HEK 293T cells to 2.0 x 108 cells. Each T225 cm2 flask grown to 100% confluency contains and average of ~2.25 x 107 cells.
- Add 1.2 L DMEM with 5% (v/v) FBS to a 10-layer cell factory. Add 2.0 x 108 HEK 293T cells to the cell factory and distribute cells evenly to all layers of the vessel. It is very difficult to visualize the confluency of cells in the cell factory. As an alternative, set up a T75 cm2 flask with an appropriate number of cells, using the same cell number to surface area ratio as performed with the 10-layer vessel. Monitor this flask for growth rates. See the associated video for instruction on handling the cell factory. Incubate overnight at 37 °C with 5% CO2 to allow for cell attachment and growth.
- Perform large-scale transfection when adherent HEK 293T cells are 70% confluent. Prepare the PEI-DNA transfection mixture (3:1 w/w PEI to DNA ratio) in a biosafety cabinet using a sterile T75 cm2 flask. Mix 0.84 mg of DNA with 84 ml of sterile 1X PBS, then add 2.5 ml of PEI (2.5 mg total PEI). Incubate at room temperature for 15 minutes. Solution should become cloudy.
- Pour the PEI-DNA transfection mixture slowly into the cell factory and thoroughly distribute over all layers of the vessel. Optional: for increased expression yields, add valproic acid (4 mM final concentration). Incubate at 37 °C with 5% CO2 for four days.
- Harvest supernatant four days post-transfection. Centrifuge the conditioned media at 6000 x g for 30 minutes at 4 °C. Further filter the supernatant using a 0.22 μm Stericup vacuum filter apparatus. The 10-layer cell factory can be reused, see Box 3 for cleaning instructions. It is key that cleaning is initiated immediately after supernatant harvest; do not let the cells dry onto the vessel surface.
- Concentrate the supernatant to 75 ml using the Centramate tangential flow filtration system.
- Add 500 ml of PBS and re-concentrate to 75 ml. Repeat three additional times to completely buffer exchange the sample.
- Equilibrate a 1 ml anti-HA affinity column with 1X PBS and apply concentrated sample by gravity flow at a rate <1 ml/min.
- Wash the column with 30 ml of 1X PBS-Tween20.
- Dissolve HA peptide in 1X PBS (1.0 mg/ml) and incubate at 37 °C.
- Apply 1 ml of the HA peptide to the anti-HA column and allow the peptide to flow into the resin. Collect the flow through. Stop the flow when the peptide solution reaches the bed height.
- Incubate the entire anti-HA column at 37 °C for 15 minutes.
- Repeat Step 12 two additional times.
- Apply 1 ml of 1X PBS to the anti-HA column and flow into the resin until it reaches the bed height. Collect the flow through.
- Regenerate the anti-HA column with 10 ml 0.1 M glycine pH 2.2. Wash with 10 ml PBS and store affinity column at 4 °C in PBS with 0.02% (w/v) NaN3.
- Perform SDS-PAGE analysis and pool the fractions accordingly. Note: The HA peptide will interfere with protein concentration measurements by A280 or Bradford. To estimate the amount of protein present, load 5, 10, 15, 25 μg of BSA onto the SDS-PAGE gel as a standard and compare the band intensities.
Steps 9-17 can be repeated to capture additional protein from the conditioned medium.
4. Representative Results
In this article, we describe and demonstrate a convenient expression platform for milligram-quantity production of human proteins that can subsequently be used for structural and functional studies. The screening of human protein constructs using HEK 293T cells in 6-well plates is efficient and effective in identifying constructs amenable to larger scale production. Commercial expression vectors can be transfected efficiently in HEK 293T cells using a variety of transfection reagents, such as GeneJuice, FuGene HD or PEI. We recommend the use of a commercial transfection reagent, such as GeneJuice or FuGene HD, for test expressions, as these reagents are more effective for poorer expressing proteins (Fig. 3). Constructs selected for larger scale expression should be characterized by a single, strong intensity band, corresponding to the proper molecular weight on the western blot (Fig. 3). Glycoproteins may migrate as a broader band due to heterogeneity in glycosylation. We have shown that a variety of macromolecules, ranging from viral glycoproteins, cytokines, cytokine receptors, and other surface proteins, can be expressed and purified to yield millgram quantities of protein using this general expression platform (Fig. 4).
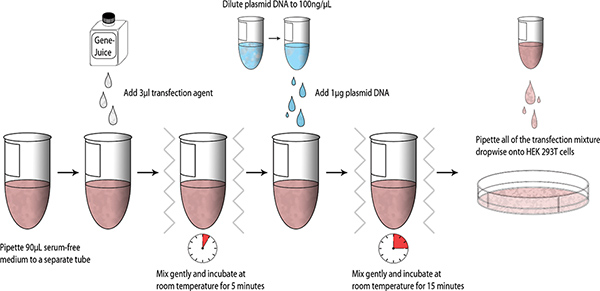
Figure 1. Workflow schematic of small-scale transfections. Click here to view larger figure.

Figure 2. Corning 10-layer CellSTACK for larger scale protein expression. Each layer contains 636 cm2 surface area for cell attachment. A standard laboratory CO2 incubator (6.0 cu. ft.) will comfortably hold four 10-layer cell factories.
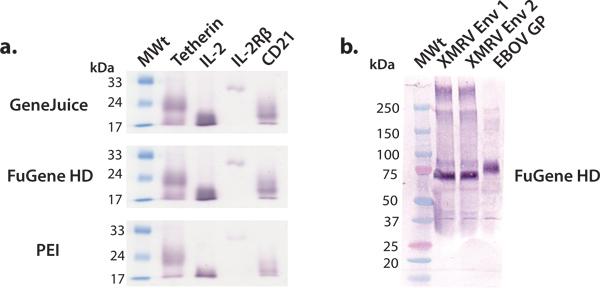
Figure 3. Small-scale expression of various secreted proteins. We performed a series of small-scale test expressions using common transfection reagents: GeneJuice, FuGene HD and PEI. (a) Western blot screening of selected human cellular proteins (tetherin), receptors (IL-2R β subunit) and cytokines (IL-2). Tetherin is a human membrane glycoprotein that restricts the release of nascent HIV-1 virions 16. The extracellular domain of tetherin exists as a glycosylated, disulfide-linked dimer of ~36 kDa. Under reducing conditions, as shown here, tetherin migrates as a monomer with an apparent molecular weight of ~22 kDa. Interleukin-2 (IL-2) is a cytokine (~17 kDa) involved in lymphocyte proliferation 17. It interacts with the IL-2 receptor complex, of which IL-2R βsubunit (~26 kDa) is a component 18. CD21 is a membrane protein involved in the activation and maturation B-cells by the complement system, and is also a receptor for the Epstein-Barr virus. The glycosylated extracellular domain of CD21 migrates as a monomer with an apparent molecular weight of ~20 kDa. (b) Western blot screening of selected surface viral glycoproteins (XMRV Env and ebolavirus GP). XMRV and ebolavirus glycoproteins (transmembrane anchor deleted) exist at the viral membrane as trimeric spikes and are involved in host cell attachment and fusion. The ectodomain of XMRV Env and EBOV GP are heavily decorated with N-linked glycans and migrate at apparent molecular weights of 70 kDa and 75 kDa, respectively.

Figure 4. Purified human cellular proteins from large-scale HEK 293T cultures. All proteins were expressed using a 10-layer cell factory, and concentrated and purified by anti-HA chromatography. As shown by Coomassie-stained SDS-PAGE analysis, the extracellular domains of the interleukin-2 receptor (IL-2R) α and γ subunits migrate at molecular weights of 40 kDa and 46 kDa, respectively. The extracellular domain of tetherin migrates as a dimer, under non-reducing conditions, with an apparent molecular weight of 36 kDa. Note that there is some BSA contamination that appears at an apparent molecular weight of 60 kDa. In addition, the heterogeneity of the N-linked glycans present on tetherin, IL-2R α and IL-2R γ causes band broadening on the SDS-PAGE gel. These complex-type N-linked glycans can be removed using peptide: N-glycosidase F.
| Vessel | Surface area |
| 6-well plate | 9.5 cm2 (each well) |
| 100 mm dishes | 55 cm2 |
| 245 mm dishes | 500 cm2 |
| T75 cm2 flask | 75 cm2 |
| T175 cm2 flask | 175 cm2 |
| T225 cm2 flask | 225 cm2 |
| Roller bottle- regular | 850 cm2 |
| Roller bottle- expanded surface | 1700 cm2 |
| 1-layer CellSTACK | 636 cm2 |
| 2-layer CellSTACK | 1272 cm2 |
| 5-layer CellSTACK | 3180 cm2 |
| 10-layer CellSTACK | 6360 cm2 |
| 40-layer CellSTACK | 25,440 cm2 |
Table 1. Comparison of cell culture vessels used for protein expression.
List of Reagent Recipes
100X Ciprofloxacine For 10 ml solution, add 10 ml deionized water to 10 mg ciprofloxacine. Add 10 μl 6N HCl to completely dissolve the ciprofloxacine.
PEI (1 mg/mL) For a 100 ml solution, dissolve 100 mg of 25 kDa linear PEI in deionized water and heat to 80 °C. Cool solution to room temperature, adjust pH to 7.2, 0.22 μm filter sterilize, aliquot and freeze at -20 °C for long-term storage.
1X PBS For 1 L aqueous solution: 8.0 g NaCl, 0.2 g KCl, 1.4 g Na2HPO4 (anhydrous), 0.24 g KH2PO4. Adjust pH of solution to 7.4 and fill to 1.0 L.
1X PBS-Tween-20 For 1 L aqueous solution: 8.0 g NaCl, 0.2 g KCl, 1.4 g Na2HPO4 (anhydrous), 0.24 g KH2PO4, 1 ml Tween-20. Adjust pH of solution to 7.4 and fill to 1.0 L.
1X transfer buffer For 1 L aqueous solution: 3.0 g Tris base, 14.4 g glycine, 150 ml methanol.
1X SDS-PAGE running buffer For 1 L aqueous solution: 3.0 g Tris base, 14.4 g glycine, 1.0 g SDS.
SDS-PAGE reducing sample buffer For 10 ml solution: 0.6 g SDS, 3 ml glycerol, 1.8 ml 1.0 Tris-HCl pH 6.8, 1 mg bromophenol blue, 5% (v/v) 2-mercaptoethanol.
Box 1. General protocols for cell propagation
- Grow HEK 293T cells in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 1X pen/strep at 37 °C in a 5% CO2-humidified atmosphere.
- Observe cells under an inverted microscope. When cells are at 100% confluency, remove and discard culture medium.
- Rinse cells with 5 ml of sterile 1X PBS to remove traces of serum. Discard the PBS wash.
- Add 2 ml of 0.05% (w/v) trypsin-EDTA solution to a T225 cm2 flask (or 1 ml of 0.05% (w/v) trypsin-EDTA for T75 cm2 flask) and incubate at room temperature until cells detach from surface. It is also possible to use sterile 1X PBS-EDTA to detach HEK 293T cells.
- Add 13 ml of DMEM with 10% (v/v) FBS to a T225 cm2 flask (or 9 ml of DMEM with 10% (v/v) FBS for T75 cm2 flask) to inhibit the trypsin reaction.
- Split cells 1:5. For a T225 cm2 flask, add 3 ml of cell suspension to 27 ml of fresh DMEM with 1X pen/strep and 10% (v/v) FBS in a new culture vessel. For a T75 cm2 flask, add 2 ml of cell suspension to 8 ml of fresh growth media. Incubate cultures at 37 °C in a 5% CO2-humidified atmosphere. Cells should grow to 100% confluency within two days.
Box 2. Western blot analysis
- Add 10 μl SDS-PAGE reducing sample buffer to 30 μl cell culture supernatant. Load samples and prestained molecular weight markers onto polyacrylamide gels, and electrophorese using 1X SDS-PAGE running buffer at 175 V for 1 hour or until molecular weight markers are well resolved.
- Soak the PVDF Immobilon-P membrane in 100% methanol for 1 minute to activate.
- Assemble western blot apparatus. Ensure the PVDF membrane faces the positive electrode and keep all components wet with 1X transfer buffer. Avoid bubbles between the polyacrylamide gel and the membrane.
- Completely fill the electrophoresis chamber with 1X transfer buffer and transfer for 1 hour at 100 V.
- Block the membrane with 5% (w/v) skim milk in 1X PBS-Tween20 for 1 hour at room temperature, or overnight at 4 °C.
- Incubate with monoclonal primary antibody (i.e. 1:1000 dilution anti-HA mAb or another appropriate antibody) dissolved in 5% (w/v) skim milk in 1X PBS-Tween20 for 1 hour at room temperature or overnight at 4 °C.
- Wash the membranes in 1X PBS-Tween20 for 10 minutes. Repeat two extra times.
- Incubate with a monoclonal secondary antibody conjugated with alkaline phosphatase (1:1000 dilution in 5% (w/v) skim milk in 1X PBS-Tween20) for 1 hour at room temperature, or overnight at 4 °C.
- Wash the membranes in 1X PBS-Tween20 for 10 minutes. Repeat two extra times.
- Place the membrane into a small container, add 5 ml alkaline phosphatase substrate (BCIP/NBT) solution. Colour development should occur within 1-5 minutes. Once desired band intensity is reached, wash with deionized water and air dry. Colour may fade over time; electronically scan the membranes once dry.
Box 3. Cleaning and recycling of cell culture vessels
While cell factories are designed to be single use, these vessels can be recycled for additional large-scale transfections using the following cleaning protocol:
- Immediately after decanting the supernatant from the 10-layer cell factory, add 20% (v/v) bleach and shake vigorously to detach cells. Incubate at room temperature for three hours.
- Empty the vessel and add fresh 20% (v/v) bleach and incubate at room temperature overnight.
- Empty the vessel and wash with 1.5 L of deionized water. Repeat three times.
- Empty the 10-layer cell factory and add 0.5 L of sterile 1X PBS supplemented with 10X Antibiotic/Antimycotic solution. Store the vessel at room temperature and replace venting caps for filling ports (standard 33 mm threaded caps) prior to next usage. Note: all layers should be completely clear after cleaning, if not, do not use and dispose the cell factory according to institutional guidelines.
Discussion
The 10-layer cell factories are an effective vessel for the production of milligram quantities of protein. A major advantage of using the cell factory over other traditional vessels, such as roller bottles, shake flasks or spinner flasks, is that they do not require the purchase of any additional laboratory equipment. A standard CO2 incubator (~6.0 cu. ft.) will easily accommodate four 10-layer cell factories (Fig. 2). In addition, these vessels require less labor and space than dishes, flasks...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by an Ontario HIV Treatment Network Research Operating Grant (ROG-G645) and Canadian Institutes of Health Research New Investigator Award (MSH-113554) to JEL, and University of Toronto Fellowships to HA, FCA, and JDC. The authors would like to thank Marnie Fusco, Dafna Abelson and Dr. Erica Ollmann Saphire at The Scripps Research Institute (La Jolla, CA) for providing cells, ebolavirus GP expression vector and general advice.
Materials
| Name | Company | Catalog Number | Comments |
| Alkaline phosphatase (BCIP/NBT) liquid substrate solution | Sigma | B6404 | |
| Antibiotic/Antimycotic, 100X | Invitrogen | 15240062 | |
| Anti-HA affinity matrix, clone 3F10 | Roche | 1815016 | |
| Anti-HA murine mAb, clone 16B12 | Covance | MMS-101P | |
| Cell culture flask, T75 cm2 tissue culture treated | Corning | 430641 | |
| Cell culture flask, T225 cm2 tissue culture treated | Corning | 431082 | |
| Cell culture plates,6-well tissue culture treated | Corning | 3516 | |
| Cell factory, 10-layer CellSTACK | Corning | 3312 | |
| Centramate Omega 5K Cassette | Pall | OS005C12 | |
| Centramate Omega 30K Cassette | Pall | OS030C12 | |
| Chromatography glass column, 1.0x10 cm | Kontes | 4204001010 | |
| Ciprofloxacin | Sigma | 17850 | |
| CO2 | |||
| Dulbecco's modified Eagle's media (DMEM) | Sigma | D5796 | |
| Fetal bovine serum (FBS), heat inactivated | Invitrogen | 12484-028 | |
| FuGENE HD transfection reagent | Promega | 4709691001 | |
| GeneJuice transfection reagent | EMD/Merck | 70967-6 | |
| Glycine | Sigma | G8898 | |
| Goat anti-mouse IgG F(ab')2 alkaline | Thermo Scientific | 31324 | |
| phosphatase-conjugated antibody | |||
| Hemagglutinin (HA) peptide, 100 mg | Genscript | custom synthesis | |
| (sequence: YPYDVPDYA; 95% purity) | |||
| HEK 293T cells | ATCC | CRL-11268 | |
| Household bleach (4% w/v sodium hypochlorite) | various brands are available | ||
| Immobilon-P PVDF membrane | Millipore | IPVH07850 | |
| MiniPrep plasmid purification kit, PureLink Quick | Invitrogen | K2100-11 | |
| MaxiPrep plasmid purification kit, PureLink HiPure | Invitrogen | K2100-07 | |
| NaN3 | Sigma | S8032 | |
| pDISPLAY expression vector | Invitrogen | V660-20 | |
| Penicillin/streptomycin (pen/strep), 100X | Invitrogen | 15140-122 | |
| Phosphate-buffered saline (PBS), sterile 1X | Sigma | D8537 | |
| Polyethyleneimine (PEI), linear 25 kDa | Polyscience | 23966 | |
| Skim milk dry powder | Carnation | ||
| Stericup-GP PES vacuum filtration unit, | Millipore | SCGPU05RE | |
| 0.22 μm, 500 ml capacity | |||
| Trypan blue | Invitrogen | 15250061 | |
| Trypsin-EDTA, 0.05% (w/v) | Invitrogen | 25300-054 | |
| Tween-20 | Sigma | P7949 | |
| Valproic acid | Sigma | P4543 | |
| Centramate tangential flow system | Pall | ||
| CO2 humidified incubator, standard 6.0 cu. ft. | various brands are available | ||
| Electrophoresis and transfer unit | various brands are available | ||
| Incubator, 37 °C | various brands are available |
References
- Celik, E., Calik, P. Production of recombinant proteins by yeast cells. Biotechnol. Adv. , (2011).
- Yokoyama, S. Protein expression systems for structural genomics and proteomics. Curr. Opin. Chem. Biol. 7, 39-43 (2003).
- Nettleship, J. E., Assenberg, R., Diprose, J. M., Rahman-Huq, N., Owens, R. J. Recent advances in the production of proteins in insect and mammalian cells for structural biology. J. Struct. Biol. 172, 55-65 (2010).
- Carlson, E. D., Gan, R., Hodgman, C. E., Jewett, M. C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. , (2011).
- Lee, J. E. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 454, 177-182 (2008).
- Bowden, T. A. Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nat. Struct. Mol. Biol. 15, 567-572 (2008).
- Evans, M. J., Hartman, S. L., Wolff, D. W., Rollins, S. A., Squinto, S. P. Rapid expression of an anti-human C5 chimeric Fab utilizing a vector that replicates in COS and 293 cells. J. Immunol. Methods. 184, 123-138 (1995).
- Ye, J. High-level protein expression in scalable CHO transient transfection. Biotechnol. Bioeng. 103, 542-551 (2009).
- Wurm, F. M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 22, 1393-1398 (2004).
- Lee, J. E., Fusco, M. H., Hessell, A. J., Burton, D. R., Saphire, E. O. Techniques and tactics used in determining the structure of trimeric, prefusion Ebola virus GP. Acta Cryst. 65, 1162-1180 (2009).
- Aricescu, A. R. Eukaryotic expression: developments for structural proteomics. Acta. Crystallogr. D Biol. Crystallogr. 62, 1114-1124 (2006).
- Aricescu, A. R., Lu, W., Jones, E. Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta. Crystallogr. D Biol. Crystallogr. D62, 1243-1250 (2006).
- Chang, V. T. Glycoprotein structural genomics: solving the glycosylation problem. Structure. 15, 267-273 (2007).
- Lee, J. E., Fusco, M. H., Saphire, E. O. An efficient platform for screening expression and crystallization of glycoproteins produced in human cells. Nature Protocols. 4, 592-604 (2009).
- Nettleship, J. E., Rahman-Huq, N., Owens, R. J. The production of glycoproteins by transient expression in Mammalian cells. Methods Mol. Biol. 498, 245-263 (2009).
- Neil, S. J., Zang, T., Bieniasz, P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 451, 425-430 (2008).
- Nakamura, Y. Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature. 369, 330-333 (1994).
- Wang, X., Rickert, M., Garcia, K. C. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 310, 1159-1163 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved