A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Protocol for Relative Hydrodynamic Assessment of Tri-leaflet Polymer Valves
In This Article
Summary
There has been renewed interest in developing polymer valves. Here, the objectives are to demonstrate the feasibility of modifying a commercial pulse duplicator to accommodate tri-leaflet geometries and to define a protocol to present polymer valve hydrodynamic data in comparison to native and prosthetic valve data collected under near-identical conditions.
Abstract
Limitations of currently available prosthetic valves, xenografts, and homografts have prompted a recent resurgence of developments in the area of tri-leaflet polymer valve prostheses. However, identification of a protocol for initial assessment of polymer valve hydrodynamic functionality is paramount during the early stages of the design process. Traditional in vitro pulse duplicator systems are not configured to accommodate flexible tri-leaflet materials; in addition, assessment of polymer valve functionality needs to be made in a relative context to native and prosthetic heart valves under identical test conditions so that variability in measurements from different instruments can be avoided. Accordingly, we conducted hydrodynamic assessment of i) native (n = 4, mean diameter, D = 20 mm), ii) bi-leaflet mechanical (n= 2, D = 23 mm) and iii) polymer valves (n = 5, D = 22 mm) via the use of a commercially available pulse duplicator system (ViVitro Labs Inc, Victoria, BC) that was modified to accommodate tri-leaflet valve geometries. Tri-leaflet silicone valves developed at the University of Florida comprised the polymer valve group. A mixture in the ratio of 35:65 glycerin to water was used to mimic blood physical properties. Instantaneous flow rate was measured at the interface of the left ventricle and aortic units while pressure was recorded at the ventricular and aortic positions. Bi-leaflet and native valve data from the literature was used to validate flow and pressure readings. The following hydrodynamic metrics were reported: forward flow pressure drop, aortic root mean square forward flow rate, aortic closing, leakage and regurgitant volume, transaortic closing, leakage, and total energy losses. Representative results indicated that hydrodynamic metrics from the three valve groups could be successfully obtained by incorporating a custom-built assembly into a commercially available pulse duplicator system and subsequently, objectively compared to provide insights on functional aspects of polymer valve design.
Introduction
Heart valve disease often results from degenerative valve calcification1, rheumatic fever2, endocarditis3,4 or congenital birth defects. When valve damage occurs, causing stenosis and/or regurgitation valve prolapse and cannot be surgically repaired, the native valve is usually replaced by a prosthetic valve. Currently available options include mechanical valves (cage-ball valves, tilting disk valves, etc.), homograft, and bioprosthetic valves (porcine and bovine valves). Mechanical valves are often recommended for younger patients based on their durability; however the patient is required to remain on anticoagulant therapy to prevent thrombotic complications5. Homograft and biological prosthetic valves have been effective choices to avoid blood thinner therapy; however, these valves have elevated risk for fibrosis, calcification, degeneration, and immunogenic complications leading to valve failure6. Tissue-engineered valves are being investigated as an emerging technology7-9, but much still remains to be uncovered. Alternative durable, biocompatible, prosthetic valves are needed to improve the quality of life of the heart valve disease patients. Again, this valve design could replace the bioprosthesis used in transcatheter valve technology, with transcatheter approaches showing the potential for transforming the treatment of selected patients with heart valve disease10.
As stated by current standards, a successful heart valve substitute should have the following performance characteristics: "1) allows forward flow with acceptably small mean pressure difference drop; 2) prevents retrograde flow with acceptably small regurgitation; 3) resists embolization; 4) resists hemolysis; 5) resists thrombus formation; 6) is biocompatible; 7) is compatible with in vivo diagnostic techniques; 8) is deliverable and implantable in the target population; 9) remains fixed once placed; 10) has an acceptable noise level; 11) has reproducible function; 12) maintains its functionality for a reasonable lifetime, consistent with its generic class; 13) maintains its functionality and sterility for a reasonable shelf life prior to implantation."11. Some of the shortcomings of existing valve prostheses may potentially be overcome by a polymer valve. Biocompatible polymers have been considered top candidates based on biostability, anti-hydrolysis, anti-oxidation, and advantageous mechanical properties such as high strength and viscoelasticity. In particular, elastomeric polymers may provide material deformation resembling native valve dynamics. Elastomers can be tailored to mimic soft tissue properties, and they may be the only artificial materials available that are bio-tolerant and that can withstand the coupled, in vivo, fluid-induced, flexural and tensile stresses, yet, move in a manner resembling healthy, native valve motion. Moreover, elastomers can be mass-produced in a variety of sizes, stored with ease, are expected to be cost-effective devices and can be structurally augmented with fibrous reinforcement.
The concept of the use of polymer materials to assemble a tri-leaflet valve is not new and has been the subject of several research investigations over the last 50 years12, which were abandoned largely due to limited valve durability. However, with the advent of novel manufacturing methodologies13,14, the reinforcement of polymer materials15,16 and potentially seamless integration of polymer valve substitutes with transcatheter valve technology, there has recently been a renewed interest and activity in developing polymer valves as a potentially viable alternative to currently available commercial valves. In this light, a protocol for enabling testing of these valves to assess hydrodynamic functionality is the first step in the evaluation process; yet commercially available pulse simulator systems generally do not come equipped to accommodate tri-leaflet valve designs and contain an annular spacing to insert commercially available heart valves (e.g. tilting disc, bi-leaflet mechanical heart valves). Secondly, polymer valves are an emerging technology whose hydrodynamics can only be assessed in a relative context. Even though native heart valve pressure and flow data is available, it is important to conduct testing of native aortic porcine valves, which are biologically similar to human valves, using the same pulsatile simulator that is used to evaluate the polymer valves so as to account for measurement differences that may be system dependent. Thus, the goal of this study was to demonstrate how a commercially available pulse simulator can be fitted with an assembly to accommodate tri-leaflet valve constructs and to systematically evaluate polymer valve hydrodynamic metrics in a relative context in comparison to mechanical and native porcine heart valve counterparts. In our case, novel tri-leaflet silicone polymer valves previously developed at the University of Florida 13 comprised the polymer valve group.
Protocol
1. Preparation
- Design and fabricate an assembly to accommodate a tri-leaflet valve geometry. This will at minimum include a valve holder to suture-in the valve leaflets and a tube to house the valve holder and surrounding accessories to secure the assembly onto the pulse duplicator system. In our case, we utilized a commercially available pulse duplicator system available from ViVitro Labs Inc. (Victoria, BC). Valve holder design as well as pre and post assembly configurations are depicted in Figure 1.
- The entire loop will need to be primed prior to usage. This involves two steps: i) cleansing of the entire loop system using soap solution and water, including replacement of any degraded tubes prior to use and ii) calibration of instruments connected to the loop, namely the pump being used, the flow probe, and the pressure transducers (generally measured at atrial, aortic and ventricular locations). Calibration can initially be performed using 1% saline solution and should be repeated prior to using blood-analog glycerin solution.
2. Native Aortic Valve Dissection
- Obtain 4 fresh pig hearts with the aorta intact from a USDA approved slaughter house (Institutional Animal Care and Use Committee (IACUC) approval may be required). In our case, our dissection protocol was approved by the IACUC at Florida International University (Protocol Approval Number: 11-020). Rinse the heart with deionized water and place it in a receptacle filled with the 1% antimycotic/antibiotic and sterile phosphate buffered saline (PBS) solution and transport on ice to the hydrodynamic testing laboratory.
- Place hearts in a dissecting pan and carefully remove the pericardium. Position the heart such that ventral side is facing you. Visually inspect and identify the four chambers of the heart and locate the aortic arch on the intact aorta.
- Separate the heart into two halves by cutting across horizontally at approximately 0.75 in below the annulus, i.e. the junction between the aorta and the left ventricle. Carefully isolate the intact aorta still attached to the left ventricular tissue segment.
- Examine the aortic valve located in the aortic root, the region between the ascending aorta and the lower annulus, ensuring that there is no damage or any signs of calcification.
- Split the aorta at ~1 in above the annulus and separate the left ventricular tissue segment below the annulus to isolate the aortic valve (Figure 2a).
3. Polymer and Native Valve Suturing Process
- Place the heart valve inside the valve holder such that the base of each valve aligns with the base of the post holder. Secure the valve in place at each post temporarily with a paper clip, but be careful not to damage the commissures or the cusps.
- Insert the suture in the needle. Begin suturing at the bottom of the valve holder by passing the needle through the first hole, from the outside to the inside such that the needle may be easily pulled from the bottom. In a looping fashion, start suturing the valve vertically up the posts of the valve holder.
- Progress with suturing (Figure 2b) along the circumference of the holder and secure with additional suture around the tips of the holder posts. Paper clips (Figure 2c) can be removed when the valve is completely secured using sutures to the 3 posts and at the circumference of the valve holder (Figures 2d and 2e).
4. Hydrodynamic Evaluation
Note: Actual protocol will vary depending on specific pulse duplicator system being used. All information caontained herein used the ViVitro Pulse Duplicator Sysytem (ViVitro Labs, Inc., Vancouver, BC).
- Bi-leaflet valve
- Set heart rate of pulse duplicator system to 70 beats/min.
- Select a flow waveform to drive the pump (in the case of the ViVitro system the S35 waveform was chosen for all hydrodynamic tests). The specific waveform utilized in our experiments is illustrated by Lim et al. (2001)17.
- Turn on amplifier and piston pump. Warm up for 15 min.
- Place bi-leaflet valve (Figure 2f) in the aortic position.
- Smear vacuum grease on all junctions of the device where leaks could occur.
- Pour glycerin/saline liquid in the atrial compartment. Note that the pulsatile duplicator system runs on 2 L of liquid with: 35%/0.7 L glycerin and 65%/1.3 L of saline solution. The saline solution is prepared using common salt well-dissolved in deionized water at a concentration of 9 mg/ml (weight/volume).
- Turn on the flow transducer that has been placed in the aortic position.
- Calibrate the pump.
- Proceed with the flow transducer calibration followed by the pressure transducers. Similarly to the pump, simply follow the instructions given by the ViVitest software (ViVitro Labs Inc.) for each flow and pressure under the calibrate tab.
- Once calibration is complete, start the pump at a low rpm until the fluid fills the aortic compartment. Check for leaks. Use additional vacuum grease if necessary.
- Turn the two stop-cocks (aortic and ventricular transducers) to open position.
- Increase the rpm of the pump until the stroke volume reaches 80 ml/beat.
- Permit the system to run for 10 min until flow has stabilized. Flow stabilization can be verified by observing the flow and pressure waveforms displayed in the screen. Low to none variation between cycles is a good indicator of system stabilization.
- In the ViVitest software select acquire mode.
- Click on collect 10 cycles.
- From the analyze mode, click on table and save the file. Also save an image of the waveforms using the photo-snap option in ViVitest.
- Native and Polymer valves
- For polymer and animal valves, follow the same steps 3.1.1 - 3.1.3 from the bi-leaflet valve instructions.
- Place the valve holder with the sutured valve inside the glass tube from the custom made assembly. Sandwich the tube with the top and bottom pieces and secure in-place with lateral screws and nuts.
- Place assembly between the aortic chamber and the original aortic valve holder.
- Continue with steps 3.1.5 - 3.1.16 from the bi-leaflet valve instructions.
5. Post Processing
- Flow and Pressure Waveforms
- Average the data collected for each of the waveforms collected, i.e. aortic pressure (AP), ventricular pressure (VP), and flow rate (Q).
- For each group of valve (polymer, porcine native aortic valve and bi-leaflet), plot the corresponding AP, VP and Q versus time relationships on the same plot.
- For the AP, superimpose normal, native aortic valve18, and bi-leaflet prosthetic valve19 plots from the literature for validation purposes.
- Hydrodynamic metrics
- For each valve tested, the following hydrodynamic metrics should be computed: a) Forward flow pressure drop and maximum transvalvular pressure (TVP), b) the aortic root mean square (RMS) forward flow rate, c) aortic forward flow, closing, leakage and total regurgitant volume, d) valve end orifice area (EOA), e) transaortic forward flow, closing, leakage and total energy losses.
- Forward flow pressure drop is computed from TVP readings and can be categorized into 3 time intervals, P: interval that starts and ends with 0 TVP, F: interval with forward flow and H: interval starting with 0 TVP and ending with 0 flow. Maximum TVP is the maximum pressure gradient recorded across the valve from the aortic and ventricular pressure readings.
- The RMS forward flow rate (Qrms) provides a useful metric for quantifying the magnitude of forward flow rate as follows:

Where 'n' is the total number of time points collected, 'Qi' is the instantaneous flow rate measurement collected in order 'i'. - The aortic forward, closing and leakage volumes are computed based on the following time intervals, Forward: beginning of forward flow through the valve (to), to the end of forward flow (t1); Closing: from t1 till the instance of valve closure (t2); Leakage: from t2 till the end of the cardiac cycle (t3). Total regurgitant volume is simply the sum of closing and leakage volumes.
- The EOA based on blood properties can be computed for the 3 intervals, P, F and H from the mean TVP during each of these periods as20:
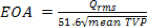
- Energy losses are defined as follows21:
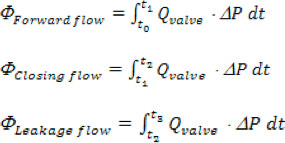
- For each valve tested, the following hydrodynamic metrics should be computed: a) Forward flow pressure drop and maximum transvalvular pressure (TVP), b) the aortic root mean square (RMS) forward flow rate, c) aortic forward flow, closing, leakage and total regurgitant volume, d) valve end orifice area (EOA), e) transaortic forward flow, closing, leakage and total energy losses.
Results
Representative flow and pressure waveforms are shown in Figures 3, 4 and 5. The plots were averaged over the sample size of valves tested for each group, which was, n = 5, 4, and 2 valves for polymer, native porcine and bi-leaflet groups, respectively. The mean hydrodynamic metrics and the standard error of the mean for these sample sizes are presented in Table 1.
Discussion
In this study, we have demonstrated the utility of modifying a commercially available pulsatile duplicator unit to accommodate tri-leaflet valve geometries so that hydrodynamic testing of polymer and native porcine valves can be performed. Specifically in our case, the system modified was a ViVitro left heart and systemic simulator system (Figure 1a) controlled via the ViViTest data acquisition system (ViVitro Systems, Inc, Victoria, BC, Canada). However, the system is not unlike several in vitro
Disclosures
The authors have nothing to disclose.
Acknowledgements
A seed grant from the University of Florida - College of Medicine is gratefully acknowledged. Graduate studies (Manuel Salinas) were supported through a minority opportunities in biomedical research programs - research initiative for scientific enhancement (MBRS-RISE) fellowship: NIH/NIGMS R25 GM061347. Financial support from the Wallace H. Coulter Foundation through Florida International University's, Biomedical Engineering Department is also gratefully acknowledged. Finally, the authors thank the following students for their assistance during various stages of the experimental process: Kamau Pier, Malachi Suttle, Kendall Armstrong and Abraham Alfonso.
Materials
| Name | Company | Catalog Number | Comments |
| Pump | ViVitro Labs | http://vivitrolabs.com/products/superpump/ | |
| Flow Meter and Probe | Carolina Medical | Model 501D | http://www.carolinamedicalelectronics.com/documents/FM501.pdf |
| Pressure Transducer | ViVitro Labs | HCM018 | |
| ViVitro Pressure Measuring Assembly | ViVitro Labs | 6186 | |
| Valve holder | WB Engineering | Designed by Florida International University. Manufactured by WB Engineering | |
| Pulse Duplicator | ViVitro Labs | PD2010 | http://vivitrolabs.com/wp-content/uploads/Pulse-Duplicator-Accessories1.pdf |
| Pulse Duplicator Data Acquisition and Control System, including ViViTest Software | ViVitro Labs | PDA2010 | http://vivitrolabs.com/products/software-daq |
| Porcine Hearts and Native Aortic Valves | Mary's Ranch Inc | ||
| Bi-leaflet Mechanical Valves | Saint Jude Medical | http://www.sjm.com/ | |
| High Vacuum Grease | Dow Corning Corporation | http://www1.dowcorning.com/DataFiles/090007b281afed0e.pdf | |
| Glycerin | McMaster-Carr | 3190K293 | 99% Natural 5 gal |
| Phosphate Buffered Saline (PBS) | Fisher Scientific | MT21031CV | 100 ml/heart |
| Antimycotic/Antibiotic Solution | Fisher Scientific | SV3007901 | 1 ml in 100 ml of PBS/heart; 20 ml for ViVitro System |
| NaCl | Sigma-Aldrich | S3014-500G | 9 g/L of deionized water |
| Deionized Water | EMD Millipore Chemicals | Millipore Deionized Purification System. 1.3 L for ViVitro System, 200 ml for heart valve dissection process |
References
- Rajamannan, N. M., et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 124, 1783-1791 (2011).
- Marijon, E., Mirabel, M., Celermajer, D. S., Jouven, X. Rheumatic heart disease. Lancet. 379, 953-964 (2012).
- Karaci, A. R., et al. Surgical treatment of infective valve endocarditis in children with congenital heart disease. J. Card. Surg. 27, 93-98 (2012).
- Knirsch, W., Nadal, D. Infective endocarditis in congenital heart disease. Eur. J. Pediatr. 170, 1111-1127 (2011).
- Korossis, S. A., Fisher, J., Ingham, E. Cardiac valve replacement: a bioengineering approach. Biomed. Mater. Eng. 10, 83-124 (2000).
- Ghanbari, H., et al. Polymeric heart valves: new materials, emerging hopes. Trends Biotechnol. 27, 359-367 (2009).
- Mol, A., Smits, A. I., Bouten, C. V., Baaijens, F. P. Tissue engineering of heart valves: advances and current challenges. Expert Rev. Med. Devices. 6, 259-275 (2009).
- Ramaswamy, S., et al. The role of organ level conditioning on the promotion of engineered heart valve tissue development in using mesenchymal stem cells. Biomaterials. 31, 1114-1125 (2010).
- Sacks, M. S., Schoen, F. J., Mayer, J. E. Bioengineering challenges for heart valve tissue engineering. Annu. Rev. Biomed. Eng. 11, 289-313 (2009).
- Zamorano, J. L., et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. J. Am. Soc. Echocardiogr. 24, 937-965 (2011).
- ANSI/AAMI/ISO. Cardiovascular Implants - Cardiac Valve Prostheses. Assoc. Adv. Med. Instrum. 71, (2005).
- Gallocher, S. L. . Durability Assessment of Polymer Trileaflet Heart Valves PhD thesis. , 313 (2007).
- Carroll, R., Boggs, T., Yamaguchi, H., Al-Mously, F., DeGroff, C., Tran-Son-Tay, R. Blood Cell Adhesion on Polymeric Heart Valves. , (2012).
- Pierre, K. K., Salinas, M., Carroll, R., Landaburo, K., Yamaguchi, H., DeGroff, C., Al-Mousily, F., Bleiweis, M., Ramaswamy, S. Hydrodynamic Evaluation of a Novel Tri-Leaflet Silicone Heart Valve Prosthesis. , (2012).
- Cacciola, G., Peters, G. W., Schreurs, P. J. A three-dimensional mechanical analysis of a stentless fibre-reinforced aortic valve prosthesis. J. Biomech. 33, 521-530 (2000).
- De Hart, J., Cacciola, G., Schreurs, P. J., Peters, G. W. A three-dimensional analysis of a fibre-reinforced aortic valve prosthesis. J. Biomech. 31, 629-638 (1998).
- Lim, W. L., Chew, Y. T., Chew, T. C., Low, H. T. Pulsatile flow studies of a porcine bioprosthetic aortic valve in vitro: PIV measurements and shear-induced blood damage. J. Biomech. 34, 1417-1427 (2001).
- Gutierrez, C., Blanchard, D. G. Diastolic heart failure: challenges of diagnosis and treatment. Am. Fam. Physician. 69, 2609-2616 (2004).
- Shi, Y., Yeo, T. J., Zhao, Y., Hwang, N. H. Particle image velocimetry study of pulsatile flow in bi-leaflet mechanical heart valves with image compensation method. J. Biol. Phys. 32, 531-551 (2006).
- Chandran, K. B., Yoganathan, A. P., Rittgers, S. E. . Biofluid Mechanics: The Human Circulation. , 277-314 (2007).
- Akins, C. W., Travis, B., Yoganathan, A. P. Energy loss for evaluating heart valve performance. J. Thorac. Cardiovasc. Surg. 136, 820-833 (2008).
- Fung, Y. C. . Biomechanics: Circulation. , (1997).
- Keener, J., Sneyd, J. . Mathematical Physiology, II: Systems Physiology. , (1998).
- Quick, C. M., Berger, D. S., Noordergraaf, A. Apparent arterial compliance. Am. J. Physiol. 274, H1393-H1403 (1998).
- Wang, Q., Jaramillo, F., Kato, Y., Pinchuk, L., Schoephoerster, R. T. Hydrodynamic Evaluation of a Minimally Invasive Heart Valve in an Isolated Aortic Root Using a Modified In Vitro Model. J. Med. Devices. 3, 011002.1-011002.6 (2009).
- Baldwin, J. T., Campbell, A., Luck, C., Ogilvie, W., Sauter, J. Fluid dynamics of the CarboMedics kinetic bileaflet prosthetic heart valve. Eur. J. Cardiothorac. Surg. 11, 287-292 (1997).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved