A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Development of Inhibitors of Protein-protein Interactions through REPLACE: Application to the Design and Development Non-ATP Competitive CDK Inhibitors
In This Article
Summary
We describe implementation of the REPLACE strategy for targeting protein-protein interactions. REPLACE is an iterative strategy involving synthetic and computational approaches for the conversion of optimized peptidic inhibitors into drug like molecules.
Abstract
REPLACE is a unique strategy developed to more effectively target protein-protein interactions (PPIs). It aims to expand available drug target space by providing improved methodology for the identification of inhibitors for such binding sites and which represent the majority of potential drug targets. The main goal of this paper is to provide a methodological overview of the use and application of the REPLACE strategy which involves computational and synthetic chemistry approaches. REPLACE is exemplified through its application to the development of non-ATP competitive cyclin dependent kinases (CDK) inhibitors as anti-tumor therapeutics. CDKs are frequently deregulated in cancer and hence are considered as important targets for drug development. Inhibition of CDK2/cyclin A in S phase has been reported to promote selective apoptosis of cancer cells in a p53 independent manner through the E2F1 pathway. Targeting the protein-protein interaction at the cyclin binding groove (CBG) is an approach which will allow the specific inhibition of cell cycle over transcriptional CDKs. The CBG is recognized by a consensus sequence derived from CDK substrates and tumor suppressor proteins termed the cyclin binding motif (CBM). The CBM has previously been optimized to an octapeptide from p21Waf (HAKRRIF) and then further truncated to a pentapeptide retaining sufficient activity (RRLIF). Peptides in general are not cell permeable, are metabolically unstable and therefore the REPLACE (REplacement with Partial Ligand Alternatives through Computational Enrichment) strategy has been applied in order to generate more drug-like inhibitors. The strategy begins with the design of Fragment ligated inhibitory peptides (FLIPs) that selectively inhibit cell cycle CDK/cyclin complexes. FLIPs were generated by iteratively replacing residues of HAKRRLIF/RRLIF with fragment like small molecules (capping groups), starting from the N-terminus (Ncaps), followed by replacement on the C-terminus. These compounds are starting points for the generation of non-ATP competitive CDK inhibitors as anti-tumor therapeutics.
Introduction
In this article, a case study of applying the REPLACE (Replacement with partial ligand alternatives using computational enrichment) strategy to convert peptidic inhibitors of protein-protein interactions into more pharmaceutically relevant molecules is described1-3. While PPIs represent a rich but underexploited source of potential drug targets, existing methodologies are largely insufficient to make these widely accessible. Current strategies including fragment based design4, high-throughput screening5 and stapled peptides6 have provided advances, however these are in many cases ineffective. As a result, more progress and more efficient approaches are required. REPLACE has been fully validated in the development of kinase inhibitors that have improved drug-like properties and have potential for further development as anti-tumor therapeutics. This strategy is exemplified in the development of non-ATP inhibitors of cell cycle CDKs and involves as follows: 1) obtaining 3D structural information on the interactions of HAKRRLIF/RRLIF with the cyclin binding groove; 2) determining the important binding determinants for peptide interaction; 3) truncation of the peptide N-terminus containing one or more binding determinants; 4) computational identification of potential small molecule alternatives (partial ligand alternatives, PLAs) for the truncated portion of the peptide and which retain key interactions of the parent peptide; 5) synthesis or commercial sourcing of PLAs predicted to bind avidly with the sub site previously occupied by the deleted peptide residue(s); 6) synthesis of FLIPs through ligation of the best PLAs to the truncated peptide using solid phase synthesis; 7) testing of FLIPs in an in vitro binding or functional assay (fluorescence polarization in the CDK/cyclin context) followed by further characterization in a cell viability assay. A schematic representation of REPLACE strategy is shown in Figure 1. In this article, iterations of the REPLACE strategy are discussed and the application to CDK2/cyclin A described in detail. CDKs are believed to be directly or indirectly deregulated in the majority of tumors and are therefore considered appropriate cancer drug targets7. CDKs require association with cyclins for full activation and subsequently phosphorylate key proteins involved in cell cycle regulation8. The two major groups of CDKs are the isotypes that control cell cycle checkpoints [G1/S (CDK4/Cyclin D, CDK6/cyclin D and CDK4/cyclin E), S phase (CDK2/cyclin A) and G2/M (CDK1/cyclin B)] and the regulators of RNA polymerase through phosphorylation (CDK7/cyclin H, CDK8/cyclin C, CDK9/cyclin T). A key step in S phase progression occurs when the E2F1 transcription factor forms a complex with the DP protein which then binds to DNA and initiates gene transcription. CDK2/cyclin A is required to neutralize E2F1 transcriptional activity through phosphorylation thereby leading to release of the E2F1-DP complex and its subsequent degradation. Inhibition of CDK2/cyclin A is believed to maintain E2F1 in its DNA bound state leading to persistent activation. The resultant level of E2F-1 activity will surpass the threshold required to induce p53 independent apoptosis therefore suggesting a therapeutic strategy. Due to deregulated p53 and pRb pathways, high levels of E2F-1 frequently occur in cancer cells and inhibition of CDK2/cyclin A should lead to selective apoptosis in tumors and can be considered as a validated cancer target7.
Clinically investigated CDK inhibitors target the highly conserved ATP binding site leading to cross reactivity among the greater than 500 protein kinases in the human kinome and potentially giving rise to side effects and toxicity9. An alternate approach is non-ATP competitive inhibition by targeting substrate recruitment through the CBG present on cyclin positive regulatory subunit and which is therefore distinct and distant from ATP binding site10,11. The CBG is primarily a hydrophobic groove present in cyclin A, cyclin D and cyclin E and has been shown to recognize a consensus sequence found in substrates and tumor suppressors. As an isolated peptide, the cyclin binding motif (CBM) binds to the CBG and has been shown to inhibit kinase activity of the cell cycle CDKs. The CBM has been optimized to an octapeptide (HAKRRLIF, CDK2/cyclin A IC50 0.07±0.02 µM , CDK4/cyclin D, IC50 0.88±0.34 µM) and furthermore truncated to a pentapeptide representing a good compromise between molecular weight for drug-likeness and potency (RRLIF, CDK2/cyclin A IC50 1.01±0.17 µM, CDK4/cyclin D, IC50 25.12±2.97 µM)12,13. The CBGs consist of a large primary and smaller secondary hydrophobic pocket which are bridged by an acidic region (includes Glu220, Glu224 and Asp283). The key binding determinants of HAKRRLIF include the interaction of Ala2 with the secondary hydrophobic pocket, ion pairing and hydrogen bonds of Lys3, Arg 4 and Arg5 with the acidic region and a high degree of complementarity of Leu6 and Phe8 with the primary lipophilic site. In addition, numerous hydrogen bonds are contributed from the peptide backbone while Ile7 acts as a spacer residue allowing optimal contact with the primary pocket. The binding mode and interactions of HAKRRLIF with CBG is shown in Figure 2.
Targeting the CBM/CBG protein-protein interaction will inhibit kinase activity of CDK2/cyclin A, CDK2/cyclin E & CDK4/cyclin D and this should trigger E2F1 mediated apoptosis of cancer cells while not affecting normal cells7. Although CBM derived peptides are effective inhibitors of cell cycle CDKs, it is unlikely that they will be useful as drugs due to their metabolic instability and general lack of cell permeability. To this end, we have applied the REPLACE strategy in order to convert these potent peptidic inhibitors into more drug-like compounds for further development of anti-tumor therapeutics exploiting deregulated E2F1 through CDK2/cyclin A inhibition. The following protocol summarizes work that has been completed in the application of REPLACE to the cyclin groove. In the first instance, drug-like capping group replacements for the N-terminal tetrapeptide of HAKRRLIF were identified. Furthermore improvements in these groups were investigated in an additional validation study for REPLACE. Representative results from these studies are also presented.
Protocol
1. Computational Identification of Potential Small Molecule Capping Groups
Note: In principle, a variety of docking or pharmacophore search methods can be used to predict potential capping groups. The main purpose of computational studies in REPLACE is to identify small molecules that retain the features and interactions of the amino acids that are substituted.
- Validation of the LigandFit docking protocol14
Note: In previous studies, the docking method (LigandFit15, a module in the molecular modeling program suite, Discovery Studio 3.0) was validated to ensure that this algorithm is sufficient to reproduce binding modes of known Ncaps and to show that the results obtained for unknown compounds are predictive14.
- Using the following steps (1.2 – 1.5), identify optimal parameters for LigandFit as follows: PLP1 energy grid for pose generation, minimization of generated poses and the PLP1 scoring function for the analysis of poses.
- In order to limit the conformations obtained and to position ligands appropriately for covalent bond formation in the capped peptide, set the indole nitrogen and amide nitrogen atoms of Trp217 and Gln254 respectively as interaction filters for hydrogen bonding.
- Design and dock a library of fragment alternatives into the CDK2/cyclin A receptor (2UUE). Prioritize potential capping groups for synthesis and commercial sourcing based on PLP1 scoring, interactions with interaction filters and complementarity with the CBG. The various steps required for LigandFit docking are as follows.
- Preparation of receptor binding site14
- Import the CDK2/cyclin A crystal structure (pdb id: 2UUE) into a visualization window in the software. This structure contains the previously identified FLIP 5-methyl-1-phenyl-1H-1,2,4-triazole-3-carboxamido(3,5-DCPT)RLIF bound to the cyclin groove (Figure 3).
- Delete or keep in place the residues RLIF (C-terminus). When the peptide sequence is retained, use the N-terminal amino group of the peptide as an interaction filter for the ligand. From the “Receptor-ligand interactions” tools, create a sphere around the N-cap 3,5-DCPT from Show/Hide site sphere. The sphere is created to define the active region in the binding site where the ligand-receptor interactions are allowed to occur during binding.
- Define the binding site further from the “find site as volume of selected ligand” and delete the ligand 3,5-DCPT from the protein. Carry out this step to define the volume of binding within the sphere created.
- Set the indole nitrogen and amide nitrogen atoms of the residues Trp217 and Gln254 as interaction filters for hydrogen bonding. Set the interaction filters so that only those poses that interact with the atoms of set residues will be filtered during the docking process therefore preserving important interactions in the docked poses.
- Preparation of ligands14
- Design a library of 100 potential capping groups based on criteria including molecular weight less than 500, presence of a carboxylate group for ligation with the truncated peptide and inclusion of appropriate hydrophobic groups (substituted phenyl group) for van der Waals interaction in the secondary pocket as shown in Table 1.
- Select heterocyclic rings for mimicking peptide hydrogen bonding interactions with Trp217 and substituents included for replacing ion-pairing interactions of basic side chains of the HAKRRLIF. Dock the ligands as respective aldehyde molecules so that the carbonyl oxygen can interact with the amide nitrogen atom of Gln254 similar to peptide backbone in the parent peptide (Table 1).
- Prior to docking, minimize designed ligands to a low energy conformation and prepare in an appropriate ionization state using the “Prepare ligands” protocol. Draw and export these potential N caps in the .sdf file format in ChemDraw for a spreadsheet prior to being imported into the software.
Note: Prepare ligands protocol is used to minimize all the ligands to be docked to their lowest energy states and the ionization charges were applied to the applicable atoms. Default parameters are used for this step.
- Docking of the ligands into the Cyclin A Groove14
- Select the LigandFit routine from receptor-ligand interactions protocol set of the software. Use the receptor and the prepared ligands as input for this protocol.
- For these runs select PLP1 as energy grid, specify that poses be energy minimized and that the number of generated poses as 10. Leave all the other parameters at the default values.
Note: LigandFit is a docking method which can identify and generate poses of high complementarity and potential affinity with the active site of a protein using a shape based comparison filter which is combined with a Monte Carlo conformational search algorithm. The energy grid used by the docking program is the force field for generating ligand-receptor interactions and potential poses. PLP1 is piecewise linear potential which specifically prioritizes hydrogen bonding interactions and where the PLP atom type is assigned for each non-hydrogen ligand atom or non-hydrogen receptor atom.
- Analysis of results
- In the first instance, display poses in the visualizer program and then sort by descending values of the PLP1 score. Use scoring functions such as PLP1 to estimate the binding affinity of a docked ligand based on a candidate ligand pose geometry and non-covalent interaction with the target receptor structure.
Note: Here, the PLP1 scoring function was found to give the best results as it includes an estimation of hydrogen bonding. - Analyze visually the top 25% of the scored poses for 1) superimposability with the known capping group (having a relative mean square deviation (RMSD) value less than 2 Å), 2) fulfilled interaction filters and 3) visual complementarity with the cyclin groove. It is accepted in that a correctly docked pose (compared to an experimental structure) has an RMSD value of < 2 Å.
- Examine poses for visual complementarity, which is defined as having efficient filling of the binding pocket in a manner that is consistent with known structure-activity relationships. Interaction filters are set to include atom restraints that require intermolecular contacts known to be critical for binding and/or are required to position the potential capping group in the correct geometry for amide bond formation. Three examples of docked poses of potential N-capping groups making hydrogen bonds with interaction filters are shown in Figure 4.
- In the first instance, display poses in the visualizer program and then sort by descending values of the PLP1 score. Use scoring functions such as PLP1 to estimate the binding affinity of a docked ligand based on a candidate ligand pose geometry and non-covalent interaction with the target receptor structure.
2. Synthesis and Characterization of Potential N-capping Groups
- Synthesis of N-capping groups.
- For synthesis, use all commercial starting materials, solvents and reagents as obtained. Synthesize potential N-capping groups by conventional synthetic organic chemistry (Figure 512,13 for an example).
- Perform thin layer chromatography (TLC) on silica gel for monitoring reactions.
- Dissolve the starting material and reaction mixture (1 mg) in the mobile phase and spot them on the TLC plate using capillary tubes.
- Place the TLC plate in to the chamber containing mobile phase (ethyl acetate and hexane at a 35:65 ratio). Once the mobile phase reaches 90% of the TLC plate, remove and air dry the plate.
- Use UV light to detect the starting material and reaction mixtures as visible spots. Calculate the Rf of all the spots (ratio of distance travelled by the spot and the mobile phase).
Note: The reaction is complete when there is no spot seen at the Rf of the starting material.
- After the completion of reaction work up the reaction mixture by placing in a separatory funnel and washing with aqueous acid or base solution as appropriate. Collect the organic solvent, evaporate it in rotary evaporator and dry the crude product obtained under vacuum.
- Dissolve 500 mg of crude product in 3-5 ml of suitable solvent and add to a 1 g of silica or RP18 samplet and dry under air. Place the samplet containing the crude product in the silica/ RP SNAP flash cartridges and purify the crude material using automated high performance flash chromatography (according to manufacturer’s protocol) employing a SNAP 100 g column with a gradient run starting from 6% ethyl acetate: 94% hexanes to 50% ethyl acetate and 50% hexanes over 15 column volumes.
- Dry the purified product collected in solvent from flash chromatography using a rotary evaporator by evaporating all the solvent to dryness and further dry the product under vacuum to remove all the residual solvents. Perform characterization of the purified product by NMR, MS and analytical HPLC.
- For 1H NMR and 13C NMR, weigh approximately 10 mg of the purified sample, dissolve it in an appropriate deuterated solvent, and transfer the contents to a dry NMR tube for recording the NMR spectra2,14.
- Record the 1H NMR and 13C NMR spectra with a high field NMR Spectrometer (minimum 300 MHz). Acquire mass spectra using a QTOF (Tandem quadruple-1 time of flight mass spectrometer), electrospray ionization (ESI) or a VG 70S (Double-focusing magnetic sector mass spectrometer, EI)2,14.
- Analyze the purity of products by HPLC with a diode-array detector and equipped with a C18 (2) 100 A, 250 x 4.6 mm, 5 µm column for analysis. Use a gradient run starting from 100% water (0.1% trifluoroacetic acid) to 60% acetonitrile (0.1% trifluoroacetic acid) over 30 min and hold the gradient for 4 min. Extract the chromatograms at 226 and 254 nm2,14.
Note: Analytical purities of evaluated compounds were > 95%.
3. Solid Phase Synthesis for the Generation of FLIPs 2
- Synthesis of N-capped FLIPs
- Assemble N-capped peptidic compounds through standard solid-phase synthesis methods using the following steps.
- Activate 5 equivalents of the C-terminal amino acid (Fmoc-Phe) in 4.4 equivalents of O-Benzotriazole-N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate (HBTU, 221.93 mg) in 2 ml of DMF for 5 min. Load the Fmoc-Phe HBTU mixture onto the Rink resin using 6 equivalents of diisopropylethyl amine (DIPEA, 103.13 mg) for 1 hr at RT.
- Remove the Fmoc protecting group from the C-terminal residue using 20% piperidine in 3 ml of DMF for 10 min. Couple subsequent amino acids (e.g., Fmoc-Ile, Fmoc-Leu, Fmoc-Arg-pmc) step by step. At each step, couple 5 equiv of the next amino acid using 6 equivalents of DIPEA (103.13 mg) and 4.4 equivalents of HBTU (221.93 mg) in 2 ml of DMF for 1 hr at RT.
- Apply wash cycles (5 x 10 ml of DMF + 5 x 10 ml of DCM) after amino acid coupling and Fmoc deprotection steps described above. After peptide assembly, couple N-capping groups using 6 equivalents of DIPEA (103.13 mg) and 4.4 equivalents of HBTU (221.93 mg) in 2 ml of DMF for 1 hr at RT.
- Upon completion of peptide assembly, treat the reaction mixture with 2 ml of the cleavage mixture (90:5:5 TFA/H2O/TIPS) O/N to remove side chain protecting groups, and cleave FLIPs from the resin. Triturate the resulting product with cold diethyl ether to precipitate and if necessary concentrate in a rotary evaporator.
- Assemble N-capped peptidic compounds through standard solid-phase synthesis methods using the following steps.
- Synthesis of N-capped-C-capped FLIPs
- Weigh and dissolve the N-terminally capped and protected Arg-Leu peptide (16.1 mg, 0.02 mmol) in dichloromethane, and add [4-(3-chlorophenoxy)pyridin-2-yl]methanamine (C-cap) along with O-benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU; 16.7 mg, 0.02 mmol) and N,N-diisopropylethylamine (DIPEA; 6.5 mg, 0.02 mmol).
- Stir and monitor the reaction at RT until TLC/HPLC indicates the complete consumption of starting material. After the completion of the reaction, concentrate the crude reaction mixture using a rotary evaporator and then partition between ethyl acetate and water.
- Separate the aqueous and organic layer; wash the organic layer with 1 N NaOH, 1 N HCl, and brine. Dry the organic layer with about 1 g of sodium sulfate and concentrate the product in rotary evaporator. Finally treat the product O/N with the cleavage mixture (95:2.5:2.5 trifluoroacetic acid/H2O/TIPS) for deprotection.
- Triturate the resulting product with cold diethyl ether and if necessary concentrate in rotary evaporator to complete dryness in order to remove all the solvent. Further dry the product under vacuum to remove all the residual solvents.
- Purification and characterization of FLIPs
- Purify crude FLIPs using semi preparative reverse-phase HPLC methods. Lyophilize the purified FLIPs and characterize their molecular mass using mass spectrometry2,14.
- Determine the analytical purity of the FLIPs by HPLC with a diode-array detector and equipped with a C18 (2) 100 A, 250 x 4.6 mm, 5 µm column. Use a gradient method starting from 95% H2O (0.1% trifluoroacetic acid)/5% acetonitrile (0.1% trifluoroacetic acid) to 35% H2O (0.1% trifluoroacetic acid)/ 65% acetonitrile (0.1% trifluoroacetic acid) over 30 min and hold the gradient for 4 min. Extract chromatograms at 226 and 254 nm.
4. Fluorescence Polarization Binding Assay for the Determination of Competitive Binding 2,14
- Perform the assay in black 384-well (138 µl) plates using the following procedure.
- Dilute the samples, tracer peptides (4 nM), and kinase complexes (CDK2/cyclin A — 18 µg/ml, CDK4/cyclin D — 37 µg/ml) to required concentrations in assay buffer (25 mM HEPES pH 7, 10 mM NaCl, 0.01% Nonidet P-40, 1 mM dithiothreitol (DTT).
- To each well of the 384-well plate, add: 5 µl of CDK4D1 or CDK2CA (0.3 µg/well purified recombinant human kinase complex), 5 µl compound solution, 5 µl of 30 nM tracer peptide (fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-(3ClPhe)-Gly or fluoresceinyl-Ahx-Pro-Val-Lys-Arg-Arg-Leu-Phe-Gly tracer peptide). Use 5 µl of each DMSO (6%), buffer, tracer and 5 µl DMSO (6%), kinase complex, tracer as control wells
- After the addition of the all the components, centrifuge the plate at 500 rpm for 1 min (41.16 x g) at RT and then incubate the plate with agitation for 45 min at RT. Using a multimode plate reader and detector fitted with 485 nm/535 nm excitation/emission filters and a dichroic mirror read the fluorescein intensity of each well in the plate.
- Calculate the relative mean polarization (mp) for all the test samples using the equation showing below. Determine IC50 values by logarithmic regression by correlating relative mps and testing concentrations.
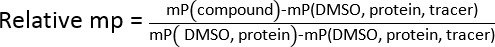
Results
The interactions of HAKRRLIF with the cyclin groove are shown in Figure 2. The peptide residues that represent the key binding determinants include Ala2, Arg4, Leu6 and Phe8 with other residues providing smaller contributions12,13,18. In this case study the REPLACE strategy has been utilized in order to find fragment alternatives for residues in the N-terminal tetrapeptide of HAKRRLIF, primarily mimicking the interactions of Ala2 and Arg4. A library of potential Ncap fragments (Table 1...
Discussion
Targeting protein-protein interactions (PPI) in drug discovery is highly challenging as these typically involve a large shallow contact interface comprised of numerous and diffuse contacts19. Furthermore, peptidic compounds which inhibit PPI’s that are amenable to drug discovery are problematic due to their higher molecular mass, metabolic instability and poor bioavailability20. Current strategies that have been applied for the development of PPI inhibitors include design of proteomimetics and...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
We thank Dr’s. Douglas Pittman and Michael Wyatt for their assistance with cell culture and Dr Wyatt and Ms. Erin Anderson for help in development of the binding assays. We acknowledge Mike Walla and Bill Cotham in the Department of Chemistry and Biochemistry at the University of South Carolina for assistance with Mass Spectrometry, Helga Cohen and Dr. Perry Pellechia for NMR spectrometry. This work was funded by the National Institutes of Health through the research project grant, 5R01CA131368.
Materials
| Name | Company | Catalog Number | Comments |
| Computational Chemistry | |||
| Accelyrs Discovery studio 3.0 | |||
| Dell Optiplex Workstations | |||
| Synthetic Organic Chemistry | |||
| Silica gel (GF-254 plates) for TLC, Biotage (Uppsala, Sweden) for flash chromatography, Waters Alliance 2695 HPLC with a 2996 diode-array detector and equipped with a C18 (2) 100 A, 250 x 4.6 mm, 5 μm column (Phenomenox Luna) for purity determination, 1H NMR and 13C NMR spectra were recorded with a Varian Mercury 300 and 400 Spectrometer, respectively. Mass spectra were measured with a Micromass QTOF (Tandem quadruple-1 time of flight mass spectrometer), electrospray ionization (ESI) and VG 70S (Double-focusing magnetic sector mass spectrometer, EI). | |||
| Flourescence Polarization Assay | |||
| 384 micro well plates, Micro pipets | Grenier Bio-one | 110256602 | |
| CDK4D1 and CDK2CA (well purified recombinant human kinase complex) | BPS Bio Sciences | 40094(CDK4/Cyclin D), 41101(CDK2/Cyclin A) | |
| assay buffer (25 nM HEPES pH 7, 10 mM NaCl, 0.01% Nonidet P-40, 1 mM dithiothretiol (DTT)) | |||
| 25 nM HEPES | CALBIOCHEM | 375368 | |
| NaCl | Fisher | 127838 | |
| Nonidet P-40 | US Biological | N3500 | |
| DTT | Aldrich | ||
| -70 °C freezer | Revco (Ultima II) | ||
| DTX880 multimode detector fitted with 485 nm/535 nm excitation/emission filters and a dichroic mirror suitable for fluorescein | Beckman Coulter, Brea, CA | ||
| Cell Culture | |||
| 96 well plates | Fisher | ||
| Frozen stocks of U2OS (osteosarcoma) and DU145 (prostate cancer) cell lines | ATCC | ||
| NU serum, DMEM media, trypsin, PEN/STRIP, MTT reagent | Fisher, Life technology, Alfa Aesar | ||
| Heamocytometer | VWR | ||
| -70 °C freezer | Revco (Ultima II) | ||
| Incubator | Thermo electron corporation | ||
| Centrifuge | Eppendorf | 5804 R | |
| Refrigerator 4-8 °C | Isotemp Fisher | ||
| DTX880 multimode detector fitted with 595 nm filter | Beckman Coulter, Brea, CA | ||
References
- Andrews, M. J., et al. REPLACE: a strategy for iterative design of cyclin-binding groove inhibitors. Chembiochem. 7, 1909-1915 (2006).
- Liu, S., et al. Optimization of Non-ATP Competitive CDK/Cyclin Groove Inhibitors through REPLACE-Mediated Fragment Assembly. J Med Chem. 56, 1573-1582 (2013).
- McInnes, C., Bernstein, M., Desai, P. Chapter 29. Annual Reports in Medicinal Chemistry. 47, 459-474 (2012).
- Bower, J. F., Pannifer, A. Using fragment-based technologies to target protein-protein interactions. Curr Pharm Des. 18, 4685-4696 (2012).
- Makley, L. N., Gestwicki, J. E. Expanding the number of 'druggable' targets: non-enzymes and protein-protein interactions. Chemical biology, and drug design. 81, 22-32 (2013).
- Walensky, L. D., Bird, G. H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J Med Chem. , (2014).
- Shapiro, G. I. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 24, 1770-1783 (2006).
- Thais, M., Sielecki, J. F. B., Enfield, P. A., Trainor, G. l. Cyclin dependant kinase inhibitors: Useful targets in cell cycle regulation. Journal of medicinal chemistry. 43, 1-18 (2000).
- Morphy, R. Selectively nonselective kinase inhibition: striking the right balance. J Med Chem. 53, 1413-1437 (2010).
- Chen, Y. N., et al. Selective killing of transformed cells by cyclin/cyclin-dependent kinase 2 antagonists. Proc Natl Acad Sci USA. 96, 4325-4329 (1999).
- Orzaez, M., Gortat, A., Mondragon, L., Bachs, O., Perez-Paya, E. ATP-noncompetitive inhibitors of CDK-cyclin complexes. ChemMedChem. 4, 19-24 (2009).
- Kontopidis, G., et al. Insights into cyclin groove recognition: complex crystal structures and inhibitor design through ligand exchange. Structure. 11, 1537-1546 (2003).
- McInnes, C., Andrews, M. J., Zheleva, D. I., Lane, D. P., Fischer, P. M. Peptidomimetic design of CDK inhibitors targeting the recruitment site of the cyclin subunit. Current medicinal chemistry. Anti-cancer agents. 3, 57-69 (2003).
- Premnath, P. N., et al. Fragment based discovery of arginine isosteres through REPLACE: towards non-ATP competitive CDK inhibitors. Bioorganic, and medicinal. 22, 616-622 (2014).
- Venkatachalam, C. M., Jiang, X., Oldfield, T., Waldman, M. LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol Graph Model. 21, 289-307 (2003).
- Mendoza, N., et al. Selective cyclin-dependent kinase 2/cyclin A antagonists that differ from ATP site inhibitors block tumor growth. Cancer Res. 63, 1020-1024 (2003).
- Denizot, F., Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 89, 271-277 (1986).
- Zheleva, D. I., et al. Highly potent p21(WAF1)-derived peptide inhibitors of CDK-mediated pRb phosphorylation: delineation and structural insight into their interactions with cyclin A. The journal of peptide research : official journal of the American Peptide Society. 60, 257-270 (2002).
- Ivanov, A. A., Khuri, K. F. R., Fu, H. Targeting protein-protein interactions as an anticancer strategy. Trends in Pharmacological Sciences. 34, (2013).
- Thayer, A. M. Improving peptides. Chemical and Engineering News. 89, 13-20 (2011).
- Yin, H., Hamilton, A. D. Strategies for targeting protein-protein interactions with synthetic agents. Angew Chem Int Ed Engl. 44, 4130-4163 (2005).
- Jubb, H., Higueruelo, A. P., Winter, A., Blundell, T. L. Structural biology and drug discovery for protein-protein interactions. Trends Pharmacol Sci. 33, 241-248 (2012).
- Cannon, J. B. Pharmaceutics and drug delivery aspects of heme and porphyrin therapy. J Pharm Sci. 82, 435-446 (1993).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved