A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A RAPID Method for Blood Processing to Increase the Yield of Plasma Peptide Levels in Human Blood
In This Article
Summary
The RAPID blood processing method can be used in humans and yields higher peptide levels as well as allows for assessment of the correct molecular form. Therefore, this method will be a valuable tool in peptide research.
Abstract
Research in the field of food intake regulation is gaining importance. This often includes the measurement of peptides regulating food intake. For the correct determination of a peptide's concentration, it should be stable during blood processing. However, this is not the case for several peptides which are quickly degraded by endogenous peptidases. Recently, we developed a blood processing method employing Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls and Dilution (RAPID) for the use in rats. Here, we have established this technique for the use in humans and investigated recovery, molecular form and circulating concentration of food intake regulatory hormones. The RAPID method significantly improved the recovery for 125I-labeled somatostatin-28 (+39%), glucagon-like peptide-1 (+35%), acyl ghrelin and glucagon (+32%), insulin and kisspeptin (+29%), nesfatin-1 (+28%), leptin (+21%) and peptide YY3-36 (+19%) compared to standard processing (EDTA blood on ice, p <0.001). High performance liquid chromatography showed the elution of endogenous acyl ghrelin at the expected position after RAPID processing, while after standard processing 62% of acyl ghrelin were degraded resulting in an earlier peak likely representing desacyl ghrelin. After RAPID processing the acyl/desacyl ghrelin ratio in blood of normal weight subjects was 1:3 compared to 1:23 following standard processing (p = 0.03). Also endogenous kisspeptin levels were higher after RAPID compared to standard processing (+99%, p = 0.02). The RAPID blood processing method can be used in humans, yields higher peptide levels and allows for assessment of the correct molecular form.
Introduction
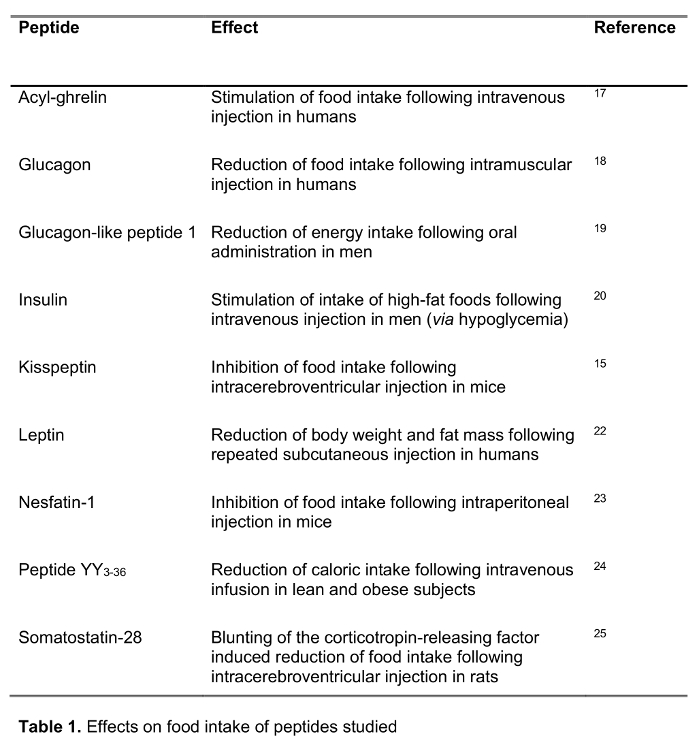
In light of the worldwide increasing prevalence of obesity1,2, research in the field of food intake regulation is gaining importance. While so far only one peptide is known that is peripherally produced and centrally acting to stimulate food intake, namely ghrelin3, within the past decades, a broad range of peptides has been identified that reduce food intake, e.g. leptin, peptide YY (PYY) and also glucagon-like peptide-1 (GLP-1) and insulin4, Therefore, in studies investigating the regulatory mechanisms of hunger and satiety peptide levels are often assessed and at the same time, it is assumed that the peptide studied is stable and recovered at high yields during plasma formation. However, very often this is not the case due to rapid endogenous breakdown as shown before for e.g. ghrelin which is degraded from acyl to desacyl ghrelin5. Therefore, we recently described the RAPID method for blood processing in rats employing Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls and Dilution6. This method improved the recovery for 11 of 12 peptides tested and allowed for the determination of the correct circulating molecular form compared to standard blood processing (EDTA blood on ice)6. This method has been used in several subsequent studies7-12 for the detection of circulating ghrelin as well as corticotropin-releasing factor13. Therefore, the method has proven useful for peptide research in rodents. However, since rodent studies are not always translatable to another species, the method should be established for the use in human blood as well.
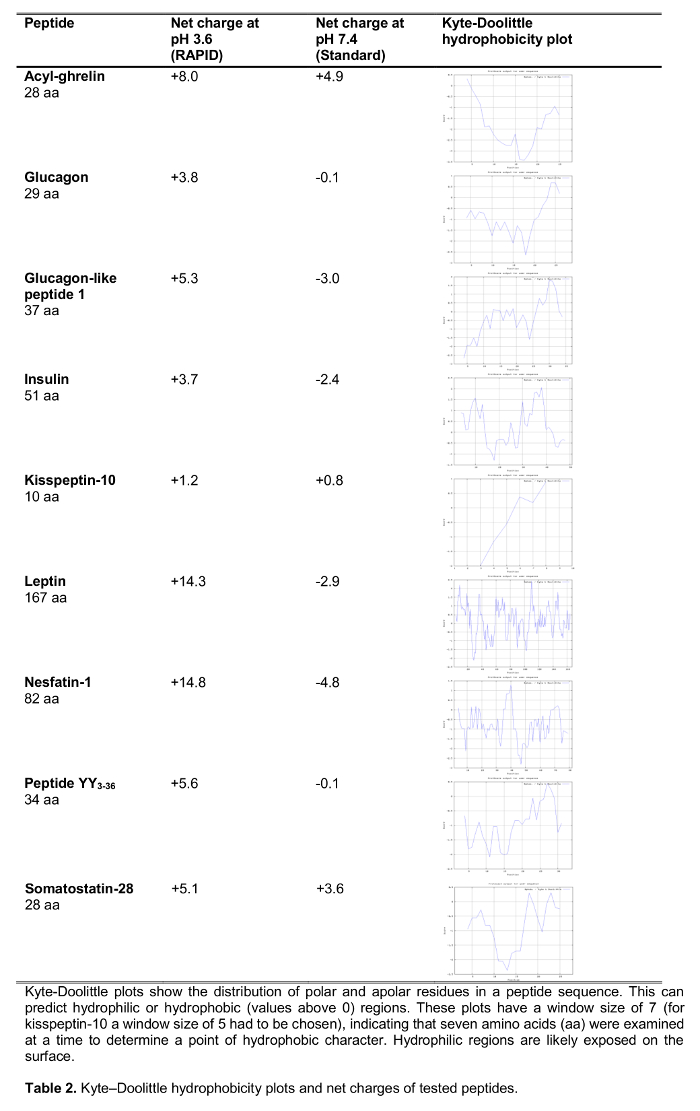
The aim of the present study was to test the RAPID method for blood processing in humans compared to standard blood processing, EDTA blood on ice, which is widely recommended14 and frequently used in the clinical as well as research setting. We tested the recovery of a selection of 125I-labeled peptides involved in the regulation of food intake including established peptides as well as new candidates recently suggested to play a role in feeding regulation (effects on food intake are shown in Table 1) following processing with both methods. Hormones were also chosen to represent peptides of different length and charge (Table 2). Moreover, for ghrelin we investigated the molecular form(s) following the standard and RAPID method. Lastly, we assessed endogenous ghrelin (acyl and desacyl ghrelin) as well as kisspeptin levels, a peptide also recently suggested to play a role in the regulation of food intake15,16 following RAPID or standard processing. In addition, we also investigated these peptide levels in a population of subjects with a wide range of body mass index (ranging from 10.2-67.6 kg/m2) to study possible differences related to chronically altered body weight.
Diagnosis, Assessment, and Plan:
Study participants
All study participants were newly hospitalized patients (inclusion was within two days of admission to the hospital) of the Division of Psychosomatic Medicine at Charité-Universitätsmedizin Berlin and gave written informed consent. To avoid any impact of gender only female patients were included. A total of 42 subjects participated in this study and were divided into three groups: normal weight (BMI 18.5-25 kg/m2, n = 12), anorexia nervosa (BMI <17.5 kg/m2, n = 15) and obesity (BMI >30 kg/m2, n = 15). Anorexic and obese patients were diagnosed according to the International Classification of Diseases-10 and hospitalized for weight gain (anorexia nervosa) or weight reduction (obesity), respectively. All normal weight patients were hospitalized exclusively due to somatoform symptoms without relevant somatic disorders. Patients with gastrointestinal somatoform symptoms or a history of gastrointestinal surgery were excluded. Exclusion criteria also encompassed an age <18 years, current pregnancy and untreated psychotic diseases. Blood collection was performed on day 2 or 3 after hospital admission before receiving dietary treatment in order to increase or reduce body weight, respectively. Anthropometric parameters were assessed on the same day.
Access restricted. Please log in or start a trial to view this content.
Protocol
The protocol was approved by the local ethics committee for human research (protocol number EA1/114/10).
1. Blood Processing
- Collect venous blood between 07:00 and 08:00 am after an overnight fast from a forearm vein and process according to standard procedure or the RAPID method. Instruct the subjects to not exercise or smoke before blood withdrawal.
- For standard processing, collect blood in chilled EDTA-containing tubes and centrifuge within 10 min at 3,000 x g for 10 min at 4 °C. Collect the supernatant and keep at -80 °C until further processing by radioimmunoassay.
- For RAPID processing, immediately dilute blood (within 1 min after blood withdrawal) 1:10 in ice-cold buffer (pH 3.6) containing 0.1 M ammonium acetate, 0.5 M NaCl and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin, chymostatin, 1 µg/ml). Then, centrifuge within 10 min at 3,000 x g for 10 min at 4 °C and collect supernatant using a pipette in polypropylene tubes as detailed before in rats6.
- Charge chromatography cartridges (360 mg, 55-105 µm) with 100% acetonitrile (rate 10 ml/min), equilibrate with 0.1% trifluoroacetate (TFA, rate 10 ml/min) and load with the supernatant at a constant rate of 1 ml/min using a syringe pump.
- Thereafter, wash cartridges with 3 ml 0.1% TFA (rate 10 ml/min) and slowly elute with 2 ml 70% acetonitrile containing 0.1% TFA (2 ml/min).
- Dry eluted samples using vacuum centrifugation and store at -80 °C until further processing by radioimmunoassay.
NOTE: Conduct all steps in polypropylene (RAPID processing) and borosilicate (radioimmunoassay) tubes that exhibit significantly lower surface binding properties and thus minimize peptide loss for most of the peptides studied before26.
2. Measurements
NOTE: Steps in this section should be performed in a laboratory certified for work with radioactive material. Standard precautions for the work with 125I should be taken.
- Recovery of Radiolabeled Peptides
- Obtain 125I-radiolabeled human peptides (e.g. acyl-ghrelin, GLP-1, glucagon, insulin, kisspeptin, leptin, nesfatin-1, PYY3-36 and somatostatin-28).
- Keep peptides in powder form until the experiment, then freshly dilute in 0.1% acetic acid (~100,000 cpm per ml).
- For standard blood processing, directly after blood withdrawal into chilled EDTA containing tubes, transfer 1 ml of blood into a tube with 50 µl of radiolabel containing 3,000-6,000 cpm (counted directly before the experiment starts).
- For RAPID processing, transfer 1 ml blood of EDTA containing blood into a tube containing 9 ml RAPID buffer (for composition see 1.3) and 500 µl radiolabel containing 30,000-60,000 cpm. Due to the 1:10 dilution use 10-times higher volume of radiolabel for RAPID processing.
- Afterwards, process samples as described in steps 1.2 to 1.3.2.
- For the recovery experiments, do not dry samples by vacuum centrifugation and do not store at -80 °C. Instead, assess recovery of radioactively labeled peptides directly afterwards using a gamma counter.
- Measure the whole supernatant in standard samples, while in RAPID samples analyze 1/10 of the total volume to obtain comparability of the amount of radiolabel used. For measurement, transfer the supernatant in tubes fitting into the gamma counter and assess the counts per minute
- As a 100% standard, use two samples with 50 µl of 125I-radiolabeled peptide that do not undergo processing. Measure at the same time with other samples as described in 2.1.7.
- Perform the experiment five to six times for each peptide.
- High Performance Liquid Chromatography of Radiolabeled Ghrelin
- Withdraw blood in chilled EDTA containing tubes and transfer 1 ml to tubes containing 200 µl radiolabeled-acyl ghrelin containing 15,000-20,000 cpm (counted directly before the experiment starts).
- For RAPID processing, transfer 1 ml blood into a tube containing 9 ml RAPID buffer (for composition see 1.3) and 200 µl radiolabeled acyl ghrelin containing 15,000-20,000 cpm.
- Afterwards, process samples as described in steps 1.2 to 1.3.2.
- For further analysis by reverse phase HPLC, directly load samples onto a stable bond C18 column (2.1 mm x 50 mm, 1.8 µm) equilibrated in 17% acetonitrile in water (both supplemented with 0.1% TFA).
- After 5 min equilibration, use a gradient from 17-40% acetonitrile to elute the sample in 40 min (flow rate of 1 ml/min).
- Collect fractions of 1 ml every minute and analyze radioactivity using a gamma counter.
- In a separate experiment, load 200 µl radiolabeled acyl ghrelin containing 15,000-20,000 cpm onto the column directly and perform HPLC as described in steps 2.2.4 to 2.2.6.
- Radioimmunoassay
- For radioimmunoassay, thaw frozen supernatants (standard processing) and vacuum dried powder (RAPID method) at room temperature.
- Immediately before radioimmunoassay, re-suspend dry RAPID samples in double distilled H2O according to the original volume of plasma (500 µl).
- Assess kisspeptin and total (including both desacyl and acyl ghrelin) as well as acyl ghrelin as described before12,27 using commercial radioimmunoassays according to the manufacturers' protocols. Use borosilicate tubes that allow stable pellet formation.
- On day one, incubate the samples with assay buffer and primary antibody (e.g. anti-ghrelin) in the dilution provided by the manufacturer for a period of 24 hr.
- On day two, add the 125I tracer (e.g. 125I-ghrelin), vortex and incubate for a period of 24 hr.
- On day three, add the precipitating reagent, vortex and incubate as recommended by the manufacturer. Then, centrifuge tubes at 3,000 x g for 20 min at 4 °C. Remove the supernatants and count radioactivity in the pellets using a gamma counter
- Calculate desacyl ghrelin as the difference of total minus acyl ghrelin. Assess the acyl/desacyl ghrelin ratio by dividing acyl by desacyl ghrelin for each individual sample.
- Process all samples – if possible – in one batch to avoid inter-assay variability. The intra-assay variability in the present experiment was <8% for kisspeptin, <7% for total and <9% for acyl ghrelin.
3. Statistical Analysis
- Determine the distribution of the data using the Kolmogorov-Smirnov test. Express data as mean ± standard error of mean (SEM).
- Assess differences between two groups by t-test. Assess differences between multiple groups by all pair-wise multiple comparison procedures (Tukey post hoc test) or two-way ANOVA followed by Holm-Sidak method.
- Consider p <0.05 significant and perform analyses using a statistics program.
Access restricted. Please log in or start a trial to view this content.
Results
RAPID blood processing increases the yield of 125I-radiolabeled peptides in human blood compared to standard blood processing.
After standard blood processing (EDTA blood on ice), the recovery of radiolabeled peptides was ~60% in 9/9 peptides (ranging from 48-68%, Figure 1A-K). RAPID processing improved the yield in all 125I-labeled peptides, namely in somatostatin-28 (+39%, Figure 1A), glucagon-like peptide-1 ...
Access restricted. Please log in or start a trial to view this content.
Discussion
We reported before that the RAPID method for blood processing improved the recovery for 11/12 peptides compared to standard blood processing in rats6. In the present study we have shown that this method is also suited for the use in humans. Following RAPID processing, the recovery for 9 of 9 125I-labeled peptides tested was improved compared to standard blood processing (EDTA blood on ice). The observed improvement ranged from 19-39% which is likely to be relevant, especially under conditions when o...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by German Research Foundation STE 1765/3-1 (A.S.) and German Ministry for Education and Research 03IPT614A (C.G.). We thank Reinhard Lommel and Petra Buße for their excellent technical support as well as Karin Johansson and Christina Hentzschel for help with organization and execution of anthropometric measurements
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Diprotin A | Peptides International, Louisville, KY, USA | IDP-4132 | |
| E-64-d | Peptides International, Louisville, KY, USA | IED-4321-v | |
| Antipain | Peptides International, Louisville, KY, USA | IAP-4062 | |
| Leupeptin | Peptides International, Louisville, KY, USA | ILP-4041 | |
| Chymostatin | Peptides International, Louisville, KY, USA | ICY-4063 | |
| Sep-Pak C18 cartridges | Waters Corporation, Milford, MA, USA | WAT051910 | 360 mg, 55-105 µm |
| Acyl-ghrelin | Millipore, Billerica, MA, USA | 9088-HK | Radioactive |
| GLP-1 | Millipore, Billerica, MA, USA | 9035-HK | Radioactive |
| Glucagon | Millipore, Billerica, MA, USA | 9030 | Radioactive |
| Insulin | Millipore, Billerica, MA, USA | 9011S | Radioactive |
| Leptin | Millipore, Billerica, MA, USA | 9081-HK | Radioactive |
| Kisspeptin-10 | Phoenix Pharmaceuticals, Burlingame, CA, USA | T-048-56 | Radioactive |
| Nesfatin-1 | Phoenix Pharmaceuticals, Burlingame, CA, USA | T-003-26 | Radioactive |
| PYY3-36 | Phoenix Pharmaceuticals, Burlingame, CA, USA | T-059-02 | Radioactive |
| Somatostatin-28 | Phoenix Pharmaceuticals, Burlingame, CA, USA | T-060-16 | Radioactive |
| ZORBAX Rapid Resolution HT SB-C18 column | Agilent Technologies, Santa Clara, CA, USA | 822700-902 | 2.1 mm x 50 mm, 1.8 µm |
| Agilent 1200 LC | Agilent Technologies, Santa Clara, CA, USA | HPLC, several components, therefore no single catalog number | |
| Kisspeptin RIA | Phoenix Pharmaceuticals, Burlingame, CA, USA | # RK-048-56 | Radioactive |
| Total ghrelin RIA | Millipore, Billerica, MA, USA | # GHRT-89HK | Radioactive |
| Active ghrelin RIA | Millipore, Billerica, MA, USA | # GHRA-88HK | Radioactive |
| SigmaStat 3.1 | Systat Software, San Jose, CA, USA | online download |
References
- Finucane, M. M., et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 377 (9765), 557-567 (2011).
- James, W. P. The epidemiology of obesity: the size of the problem. J Intern Med. 263 (4), 336-352 (2008).
- Stengel, A., Taché, Y. Gastric peptides and their regulation of hunger and satiety. Curr Gastroenterol Rep. 14 (6), 480-488 (2012).
- Hussain, S. S., Bloom, S. R. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes (Lond). 37 (5), 625-633 (2013).
- Hosoda, H., et al. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples). Clin Chem. 50 (6), 1077-1080 (2004).
- Stengel, A., et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 150 (11), 5113-5118 (2009).
- Stengel, A., et al. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: Potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 31 (9), 1689-1696 (2010).
- Stengel, A., et al. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides. 31, 2229-2235 (2010).
- Stengel, A., et al. Central administration of pan-somatostatin agonist ODT8-SST prevents abdominal surgery-induced inhibition of circulating ghrelin, food intake and gastric emptying in rats. Neurogastroenterol Motil. 23 (7), e294-e308 (2011).
- Stengel, A., et al. Abdominal surgery inhibits circulating acyl ghrelin and ghrelin-O-acyltransferase levels in rats: role of the somatostatin receptor subtype 2. Am J Physiol Gastrointest Liver Physiol. 301, G239-G248 (2011).
- Wang, L., et al. Intravenous injection of urocortin 1 induces a CRF2 mediated increase in circulating ghrelin and glucose levels through distinct mechanisms in rats. Peptides. 39, 164-170 (2013).
- Goebel-Stengel, M., Stengel, A., Wang, L., Taché, Y. Orexigenic response to tail pinch: role of brain NPY(1) and corticotropin releasing factor receptors. Am J Physiol Regul Integr Comp Physiol. 306 (3), R164-R174 (2014).
- Goebel, M., Stengel, A., Wang, L., Reeve, J., Taché, Y. Lipopolysaccharide increases plasma levels of corticotropin-releasing hormone in rats. Neuroendocrinology. 93 (3), 165-173 (2011).
- Banfi, G., Salvagno, G. L., Lippi, G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med. 45 (5), 565-576 (2007).
- Stengel, A., Wang, L., Goebel-Stengel, M., Taché, Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport. 22 (5), 253-257 (2011).
- De Bond, J. A., Smith, J. T. Kisspeptin and energy balance in reproduction. Reproduction. 147 (3), R53-R63 (2014).
- Wren, A. M., et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 86 (12), 5992(2001).
- Schulman, J. L., Carleton, J. L., Whitney, G., Whitehorn, J. C. Effect of glucagon on food intake and body weight in man. J Appl Physiol. 11 (3), 419-421 (1957).
- Steinert, R. E., Poller, B., Castelli, M. C., Drewe, J., Beglinger, C. Oral administration of glucagon-like peptide 1 or peptide YY 3-36 affects food intake in healthy male subjects. Am J Clin Nutr. 92 (4), 810-817 (2010).
- Dewan, S., et al. Effects of insulin-induced hypoglycaemia on energy intake and food choice at a subsequent test meal. Diabetes Metab Res Rev. 20 (5), 405-410 (2004).
- Schlogl, M., et al. Increased 24-hour ad libitum food intake is associated with lower plasma irisin concentrations the following morning in adult humans. Appetite. 90, 154-159 (2015).
- Heymsfield, S. B., et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 282 (16), 1568-1575 (1999).
- Shimizu, H., et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 150, 662-671 (2009).
- Batterham, R. L., et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 349 (10), 941-948 (2003).
- Shibasaki, T., et al. Antagonistic effect of somatostatin on corticotropin-releasing factor-induced anorexia in the rat. Life Sci. 42 (3), 329-334 (1988).
- Goebel-Stengel, M., Stengel, A., Taché, Y., Reeve, J. R. Jr The importance of using the optimal plasticware and glassware in studies involving peptides. Anal Biochem. 414 (1), 38-46 (2011).
- Smets, E. M., et al. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn. 28 (4), 299-303 (2008).
- Kojima, M., et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 402 (6762), 656-660 (1999).
- Stengel, A., Yin Taché, Y., Yang, the Gastric X/A-like Cell as Possible Dual Regulator of Food Intake. J Neurogastroenterol Motil. 18 (2), 138-149 (2012).
- Inhoff, T., et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 29, 2159-2168 (2008).
- Hirayama, H., et al. Contrasting effects of ghrelin and des-acyl ghrelin on the lumbo-sacral defecation center and regulation of colorectal motility in rats. Neurogastroenterol Motil. 22 (10), 1124-1131 (2011).
- Horikoshi, Y., et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 88 (2), 914-919 (2003).
- Yang, Y. U., Xiong, X. Y., Yang, L. I., Xie, L., Huang, H. Testing of kisspeptin levels in girls with idiopathic central precocious puberty and its significance. Exp Ther Med. 9 (6), 2369-2373 (2015).
- Hosoda, H., Kojima, M., Matsuo, H., Kangawa, K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 279 (3), 909-913 (2000).
- Raff, H. Total and active ghrelin in developing rats during hypoxia. Endocrine. 21 (2), 159-161 (2003).
- Evans, M. J., Livesey, J. H., Ellis, M. J., Yandle, T. G. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 34 (2), 107-112 (2001).
- Nabuchi, Y., Fujiwara, E., Kuboniwa, H., Asoh, Y., Ushio, H. The stability and degradation pathway of recombinant human parathyroid hormone: deamidation of asparaginyl residue and peptide bond cleavage at aspartyl and asparaginyl residues. Pharm Res. 14 (12), 1685-1690 (1997).
- White, A., Handler, P., Smith, E. L. Principles of Biochemistry. , 5th ed, McGraw-Hill Book Co. New York. (1973).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved