A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
The Effect of the Application of Thyme Essential Oil on Microbial Load During Meat Drying
In This Article
Summary
Microorganisms such as Escherichia coli that contaminate meat products cause foodborne illnesses. The use of essential oils in the meat drying process has not been deeply studied. Here, we present a novel method of applying thyme essential oil to meat during drying to reduce the microbial load in dried meat.
Abstract
Meat is a high protein meal that is used in the preparation of jerky, a popular food snack, where preservation and safety are important. To assure food safety and to extend the shelf life of meat and meat products, the use of either synthetic or natural preservatives have been applied to control and eliminate foodborne bacteria. A growing interest in the application of natural food additives for meat has increased. Microorganisms, such as Escherichia coli, contaminate meat and meat products, causing foodborne illnesses. Therefore, it is necessary to improve the meat conservation process. However, the use of essential oils when the meat is being dried has not been deeply studied. In this regard, there is an opportunity to increase the value of dried meat and reduce the risk of foodborne illnesses by applying essential oils during the drying process. In this protocol, we present a novel method of applying thyme essential oil (TEO) during meat drying, specifically in vapor form directly in a drying chamber. For evaluation, we use Minimal Inhibitory Concentration (MIC) to detect the number of harmful bacteria in the treated samples compared to raw samples. The preliminary results show that this method is a viable and alternative option to synthetic preservatives and that it significantly reduces microbial load in dried meat.
Introduction
Drying as a traditional method to preserve foods has been used since ancient times. Nowadays, there is a growing interest in drying as an effective method for food preservation1,2,3. It is used to make a variety of specially processed meats. One of the most well-known is jerky.
Jerky, one of the oldest methods for meat preservation, is based on curing and drying to lower water activity and therefore to extend its shelf life4. Nowadays, jerky as a preserved cured meat is still very popular, where food safety, flavor, and texture are essential. Jerky preparation can be used for almost any type of meat, including beef, pork, poultry, or game5, and it requires chopping the meat in lean strips and drying it. Usually, marinating the meat in a curing solution or smoking are used along with drying to give the jerky its characteristic flavor6.
Despite the vast interest of drying to truly preserve food, the risk of foodborne outbreaks by E. coli from poorly dried meat is critical and needs to be controlled. There are some studies reporting foodborne gastroenteritis outbreaks particularly with E. coli O157:H7, attributed to inadequate heat processing during home-drying. Similar cases have occurred even in commercially prepared jerky7,8,9. Levine et al.10 proposed that foodborne microorganisms can survive moderate drying conditions (approximately 60 °C) used by commercial jerky producers. E. coli O157:H7 outbreaks of foodborne diseases in the middle of the 1990s were attributed to ground dried meat products6,11. Interestingly, in all the previous cases, the main risk is caused by bacterial pathogens recognized as viable but non-culturable (VBNC). Under various stresses such as temperature changes or starvation, the E. coli cells could enter a particular state known as the VBNC state12,13. The VBNC cells may then be resuscitated back to culturable cells by exposure to suitable conditions and then present a threat to human health due to foodborne contamination14,15. This means that if the meat is consumed immediately after drying the product it is safe. However, in the case of inadequate storage, such as increased humidity, there is a high risk of reactivation of pathogens and microbial growth.
Besides drying and marinade methods, there is a high demand from consumers to use natural products as an alternative to additives to improve food quality16,17. There has been a particular interest in the application of natural food additives for meat instead of classical synthetic preservatives18,19,20,21. Even though there is a lack of sufficient experimental evidence in the use of essential oils when drying the meat, early research in this field already demonstrates positive results22,23.
Since the Middle Ages, people have recognized Essential Oil Compounds (EOCs) for their antimicrobial, insecticidal, and antiparasitic chracteristics24,25,26. Today, EOCs are part of one of the most important group of bioactive natural compounds. Among the different EOCs, thymol is one of the most well-known. It is composed of more than 85% of TEO23. This phenol prevents microbial and chemical deterioration when added to food. Additionally, its antibacterial properties might be improved in combination with other natural preservatives2,27,28,29,30. Nowadays, thyme (Thymus vulgaris), a herb that belongs to Lamiaceae family, has been recognized as a flavoring agent as well as a very effective meat preservative31. A study by García-Díez et al.30 on meat products found that TEO displayed a wider inhibition pattern against foodborne pathogens when compared to other essential oils. Therefore, there is an opportunity to increase the value of dried meat and reduce the risk of foodborne illnesses by applying essential oils during the drying process.
In this protocol, we present a novel method of applying TEO during meat drying, specifically using it in vapor form directly in a drying chamber. For evaluation, we use the MIC to determine the absence of pathogenic bacteria in treated samples compared with raw ones. The preliminary results show that this method is a highly effective alternative to synthetic preservatives and that it significantly reduces microbial load in dried meat.
Protocol
1. Meat Preparation
- Obtain a short loin of beef (fresh beef from biceps femoris) from a local butchery and transfer it to the lab.
NOTE: It is recommended to transport the loin of beef at ambient temperature (20 - 25 °C), for a period not longer than 20 min in a hermetic sealed bag. - To sterilize the outer surface of the beef muscle, in a laminar safety cabinet, wash the muscle by spraying with 70% (v/v) ethanol for 10 s using a squeeze bottle of 500 mL. Apply 0.025 g of ethanol per 1 cm2 of muscle surface.
- Remove the outer surface of the meat aseptically with a knife to avoid ethanol remaining in the muscle interior. Remove approximately 3 mm of the muscle interior to keep the muscle's surface uniformity.
- Package the muscle in a plastic sealed bag and transfer it to a freezer.
- Store the muscle at -18 °C for 1 day. Then, thaw the frozen muscle at 4 °C for 6 h.
NOTE: For thawing, it is recommended to move the muscle from the freezer to the fridge. - In a laminar safety cabinet, slice each muscle into 0.5 cm thick slices with a meat cutter. Then, with a knife cut it into small 5 × 2.5 cm2 rectangular samples.
- Package the rectangular meat samples in plastic bags and store them in the freezer at -18 °C for later use.
2. Preparation of Standardized Inoculum and Inoculation Procedure in a Laminar Safety Cabinet
- Prepare standardized inoculum (1.5 × 108 CFU/mL) of E. coli ATCC 25922 to inoculate the meat samples.
- For the preparation of the stock inoculum, first dispense the lyophilized bacterial culture (delivered by the supplier) into a 15-mL sterilized tube pre-filled with 10 mL of sterilized Buffered Mueller Hinton Broth (BMHB). Cultivate this suspension for 24 h at 37 °C.
- Prepare the bacterial stock solution as follows: Take approximately 0.1 - 0.2 mL of bacterial suspension and dilute into a 20-mL vial closed with a rubber stopper with an aluminum cap pre-filled with 15 mL of sterilized BMHB. Cultivate this suspension for 24 h at 37 °C.
- Store inside the refrigerator at 4 °C for the preparation of the standardized inoculum.
- From the stock solution (see step 2.1.1) of E coli take approximately 0.1 - 0.2 mL of bacterial suspension and dilute in a 15-mL plastic sterilized tube pre-filled with 10 mL of sterilized Buffered Mueller Hinton Broth (BMHB). Incubate the tube at 37 °C for 24 h.
- For the preparation of the standardized inoculum (1.5 × 108 CFU/mL), add small amounts of this suspension in a 15-mL sterilized tube pre-filled with 10 mL of sterilized BMHB.
- Thoroughly vortex the mixture and measure the optical density (OD) at 600 nm by a densitometer32.
- Repeat steps 2.1.3 - 2.1.4 until the OD expressed as the McFarland value is increased by 0.5 compared to the value of clean BMHB.
- For the preparation of the stock inoculum, first dispense the lyophilized bacterial culture (delivered by the supplier) into a 15-mL sterilized tube pre-filled with 10 mL of sterilized Buffered Mueller Hinton Broth (BMHB). Cultivate this suspension for 24 h at 37 °C.
- For the inoculation procedure, place the rectangular meat samples in two different aluminum foils (20 cm x 30 cm), one for the control samples and the second for the inoculated meat samples.
- Over the second aluminum foil, inoculate the raw rectangular meat samples with 800 µL of bacteria suspension of the selected strain (this corresponds to 1.2 × 108 CFU per meat sample) by evenly distributing the inoculum on the surface.
- Pipette 400 µL on one side of the sample and gently spread using a sterile cell spreader on the surface. Let them dry for 10 min. Repeat the same procedure for the rest of the suspension on the other side of the sample.
- Over the second aluminum foil, inoculate the raw rectangular meat samples with 800 µL of bacteria suspension of the selected strain (this corresponds to 1.2 × 108 CFU per meat sample) by evenly distributing the inoculum on the surface.
3. Drying and TEO Application
- Transfer both aluminum foils containing the rectangular meat samples from the laminar safety cabinet to the dryer: cover each with aluminum foil, and then place the samples inside the dryer.
- Carry out the drying in a standard laboratory dryer.
NOTE: First, preheat the oven to 55 °C. This procedure can last for 20 min.- Dry the control samples for 6 h at 55 °C, with drying air relative humidity values ranging from 30 - 45%.
NOTE: Drying air relative humidity values vary in time depending on the rate of evaporation of liquid from the meat.
- Dry the control samples for 6 h at 55 °C, with drying air relative humidity values ranging from 30 - 45%.
- Calculate the volume of TEO applied, and express the essential oil concentration as a volume of TEO per dryer volume (mL/L air). For example, the dose of 1.5 mL of TEO in 53 L (volume of the dryer) results in a concentration of 0.028 mL/L air. To determine the MIC of TEO for E. coli, use doses of 1.5 mL (0.028 mL/L air), 1 mL (0.019 mL/L air), and 0.75 mL (0.014 mL/L air).
- Before drying, for the application of the TEO vapors (with thymol as the main compound 79%), soak a filter paper (12 cm x 20 cm) with a 1.5 mL dose of TEO and place into the dryer in front of the fan.
- Dry the TEO treated meat samples using the same procedure as for the control samples (steps 3.1 and 3.2).
NOTE: After the drying process ends and samples are removed, switch on the oven for 3 h at 80 °C and set the air valve indication to 100% air vent in order to clean the essential oil residues from the oven.
4. Microbial Analysis
- Before the meat inoculation with bacteria, examine the meat samples for any adulteration. The appearance of slime and the detection of any strong and pungent smells are indicative of meat spoilage. If the texture feels slimy, the bacteria may have started to multiply on the surface of the meat.
- To assess the inoculation efficiency, test the raw inoculated samples for the presence of E. coli ATCC 25922 and compare them with non-inoculated control samples before the drying procedure. For this purpose:
- Wash each meat sample (2 control samples and 2 inoculated samples). Suspend each meat sample in a sterilized flask with buffered peptone water (8.5 g of NaCl, 1 g of peptone, 5 tablets of phosphate-buffered saline, and 1 g of Polysorbate 80 in 1 L of water) in a ratio of 1:10 (w/v) with a pH range from 7 - 7.3. Shake using a shaker at 140 rpm for 10 min at room temperature.
NOTE: Wash immediately after the inoculation procedure. - Evaluate the number of bacteria by an adjusted 6 × 6 drop plate procedure summarized by Chen, Nace, and Irwin33 on Plate Count Agar (PCA) and MacConkey Agar (MCA).
NOTE: The 6 × 6 drop method uses the broth micro dilution method to prepare 10-fold serial dilutions of the investigated sample with a multichannel pipette, which is less labor intensive and more economical compared to the conventional method33,34. - Cultivate the 10-fold serial sample dilutions by the 6 × 6 drop plate procedure for the evaluation of E. coli.
NOTE: Particularly for the 6 × 6 drop method, for cultivation use six 5 µL-drops, from six selected dilutions of the investigated sample with a multichannel pipette. On appropriately dried Petri dishes, the drops absorb quickly into the agar and the planting by this method is very convenient and manageable34. - Incubate the Petri dishes at 37 °C for 24 h. After the cultivation period, evaluate the number of colonies of E. coli on the Petri dishes (CFU g-1 of dried meat) as described in section 5.
- Wash each meat sample (2 control samples and 2 inoculated samples). Suspend each meat sample in a sterilized flask with buffered peptone water (8.5 g of NaCl, 1 g of peptone, 5 tablets of phosphate-buffered saline, and 1 g of Polysorbate 80 in 1 L of water) in a ratio of 1:10 (w/v) with a pH range from 7 - 7.3. Shake using a shaker at 140 rpm for 10 min at room temperature.
- After the drying, take two inoculated dried samples and compare them with two dried non-inoculated control samples for viable E. coli, respectively. To determine the presence or absence of E. coli of these four samples, carry out the pre-enrichment process of each meat sample as follows:
- Suspend each meat sample in a sterilized flask with buffered peptone water (see step 4.2.1) and shake using a shaker at 140 rpm for 10 min at room temperature. Then incubate each flask at 37 °C during 6 h for pre-enrichment.
- For the evaluation and cultivation of the bacteria, follow the same procedure as described in steps 4.2.2 - 4.2.4.
5. Review Results
- After the incubation is complete, remove the Petri dishes from the incubator and review the results as follows:
- To evaluate the total number of colonies, examine the plates for the presence of mesophilic aerobic bacteria on PCA (white spots) and typical E. coli colonies (red to dark pink) on MCA. If the pathogen is absent, both agars present no growth.
- Count the colonies and determine the amount of E. coli (CFU/g-1 of dried meat) present.
NOTE: Count the number of colonies (N) at two consecutive dilutions containing 30 or less colonies per drop (Figure 1). The number N of CFU/g-1 of dried meat is determined as follows35
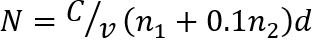
where, C is the sum of colonies on all drops counted, v is the volume of sample dilution used per drop (here, 0.05 mL), n1 is the number of drops used at the first dilution, n2 is the number of drops used at the second dilution, and d represents the dilution from which the first counts were captured.
- To analyze the microbiological data, convert the number of colonies to log CFU g-1 and subject them to analysis of variance (ANOVA) for the main effects of treatment36.
- Perform the Tukey Honest Significant Difference test (Tukey HSD) for multiple mean comparisons36 and determine the significant differences between treatments.
Results
We had first previously developed this method by using oregano essential oil (OEO) to enhance food safety and increase the value of dried meat. In general, the preceding experiments showed that E. coli goes into the VBNC state during drying as a survival strategy. This is demonstrated by the fact that there were no culturable bacteria after the drying finished22. Therefore, the pre-enrichment process for 6 h was necessary to allow the counting of the strai...
Discussion
Previous research has shown that microorganisms causing foodborne diseases survive drying10. It is therefore necessary to apply preservatives before drying to assure food safety. In this study, we focus on using TEO. The reason is twofold: First, there is a high demand from consumers to use natural products as alternative additives to improve food quality16; Second, a previous study demonstrated positive results after using OEO during the meat drying process
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Internal Grant Agency of the Faculty of Tropical AgriSciences, (project number: 20175013) and the CIGA 20182023 both grants, from the Czech University of Life Sciences.
Materials
| Name | Company | Catalog Number | Comments |
| Meat cutter | Kalorik | KP 3530 | from Miami Gardens, FL, USA |
| Laminar safety cabinet | Faster s.r.l | from Italy | |
| Squeeze bottle of 500 mL | Merci | 632 524 325 025 | from CZ |
| Standard laboratory drier UFE 400 | Memmert | DE 66812464 | from Germany |
| Incubator | BT 120 | N/A | from CZ |
| Refrigerator and Freezer | Bosch | KGN34VW20G | from DE |
| Densitometer | Biosan | 220 000 050 122 | Latvia; supplier Merci, CZ |
| Escherichia coli ATCC 25922 | Oxoid | CL7050 | from CZ |
| Vortex | Chromservis | 22008013 | from CZ |
| Sterilized plastic tubes 15 mL | Gama | 331 000 020 115 | from CZ, supplier Merci |
| 20 mL injection vial | Healthy vial | hvft169 | from China |
| 20 mm sterile butyl rubber stopper | Merci | 22008013 | from CZ |
| 20 mm aluminum cap | Healthy vial | N/A | from China |
| Thyme essential oil | Sigma Aldrich | W306509 | from St Louis, MO, USA |
| Mueller Hinton Broth | Oxoid | CM0337 | from CZ |
| NaCl | Penta | 16610-31000 | from CZ |
| Peptone | Oxoid | LP0034 | from CZ |
| Phosphate-buffered saline | Sigma Aldrich | P4417 | from CZ |
| Polysorbate 80 (Tween 80) | Roth | T 13502 | from DE, supplier P-lab |
| Shaker SHO-1D | Verkon | DH.WSR04020 | from CZ, 10 - 300 rpm. 350 x 350 mm with a platform for flasks |
| Ethanol 70% | Bioferm | N/A | from CZ |
| MacConkey Agar | Oxoid | CM007 | from CZ |
| Plate Count Agar | Oxoid | CM0325 | from CZ |
| Filter paper | Merci | 480 622 080 040 | from CZ |
| Erlenmeyer flasks 250 mL | Simax | 610 002 122 636 | from CZ; supplier Merci CZ |
| Multichannel pipette | Socorex | S852820 | from Switzerland; supplier P lab, CZ |
| Microtiter plate | Gamma | V400916 | CZ |
| Microlitre pipette 100-1000 μL | Eppendorf | 333 120 000 062 | from Germany; supplier Merci, CZ |
References
- Eklund, M. W., Peterson, M. E., Poysky, F. T., Paranjpye, R. N., Pelroy, G. A. Control of bacterial pathogens during processing of cold-smoked and dried salmon strips. J. Food Prot. 67 (2), 347-351 (2004).
- Mahmoud, B. S. M., et al. Preservative effect of combined treatment with electrolyzed NaCl solutions and essential oil compounds on carp fillets during convectional air-drying. Int. J. Food Microbiol. 106 (3), 331-337 (2006).
- Rahman, M. S., Guizani, N., Al-Ruzeiki, M. H., Al Khalasi, A. S. Microflora Changes in Tuna Mince During Convection Air Drying. Dry. Technol. 18 (10), 2369-2379 (2000).
- Faith, N. G., et al. Viability of Escherichia coli O157: H7 in ground and formed beef jerky prepared at levels of 5 and 20% fat and dried at 52, 57, 63, or 68 C in a home-style dehydrator. Int. J. Food Microbiol. 41 (3), 213-221 (1998).
- Hierro, E., De La Hoz, L., Ordóñez, J. A. Headspace volatile compounds from salted and occasionally smoked dried meats (cecinas) as affected by animal species. Food Chem. 85 (4), 649-657 (2004).
- Nummer, B. A., et al. Effects of Preparation Methods on the Microbiological Safety of Home-Dried Meat Jerky. J. Food Prot. 67 (10), 2337-2341 (2004).
- Greig, J. D., Ravel, A. Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Food Microbiol. 130 (2), 77-87 (2009).
- Eidson, M., Sewell, C. M., Graves, G., Olson, R. Beef jerky gastroenteritis outbreaks. J. Environ. Health. 62 (6), 9-13 (2000).
- Allen, K., Cornforth, D., Whittier, D., Vasavada, M., Nummer, B. Evaluation of high humidity and wet marinade methods for pasteurization of jerky. J. Food Sci. 72 (7), (2007).
- Levine, P., Rose, B., Green, S., Ransom, G., Hill, W. Pathogen testing of ready-to-eat meat and poultry products collected at federally inspected establishments in the United States, 1990 to 1999. J. Food Prot. 64 (8), 1188-1193 (1990).
- Keene, W. E., et al. An outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meat. JAMA. 277 (15), 1229-1231 (1997).
- Oliver, J. D. The viable but nonculturable state in bacteria. J. Microbiol. 43, 93-100 (2005).
- Oliver, J. D. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 34 (4), 415-425 (2010).
- Khamisse, E., Firmesse, O., Christieans, S., Chassaing, D., Carpentier, B. Impact of cleaning and disinfection on the non-culturable and culturable bacterial loads of food-contact surfaces at a beef processing plant. Int. J. Food Microbiol. 158 (2), 163-168 (2012).
- Li, L., Mendis, N., Trigui, H., Oliver, J. D., Faucher, S. P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 5, 258 (2014).
- Hernández, H., Claramount, D., Kučerová, I., Banout, J. The effects of modified blanching and oregano essential oil on drying kinetics and sensory attributes of dried meat. J. Food Process. Preserv. , (2016).
- García-Díez, J., et al. The Impact of Essential Oils on Consumer Acceptance of Chouriço de vinho - A Dry-Cured Sausage Made from Wine-Marinated Meat - Assessed by the Hedonic Scale, JAR Intensity Scale and Consumers' "Will to Consume and Purchase.". J. Food Process. Preserv. 41 (4), (2017).
- Govaris, A., Solomakos, N., Pexara, A., Chatzopoulou, P. S. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 137 (2-3), 175-180 (2010).
- Holley, R. A., Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 22 (4), 273-292 (2005).
- Petrou, S., Tsiraki, M., Giatrakou, V., Savvaidis, I. N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 156 (3), 264-271 (2012).
- Ballester-costa, C., Sendra, E., Viuda-martos, M. Assessment of Antioxidant and Antibacterial Properties on Meat Homogenates of Essential Oils Obtained from Four Thymus Species Achieved from Organic Growth. Foods. 6 (8), 59 (2017).
- Hernández, H., et al. The effect of oregano essential oil on microbial load and sensory attributes of dried meat. J. Sci. Food Agric. 97 (1), 82-87 (2017).
- García-Díez, J., Alheiro, J., Falco, V., Fraqueza, M. J., Patarata, L. Chemical characterization and antimicrobial properties of herbs and spices essential oils against pathogens and spoilage bacteria associated to dry-cured meat products. J. Essent. Oil Res. 29 (2), 117-125 (2017).
- Cavanagh, H. M. A. Antifungal Activity of the Volatile Phase of Essential Oils: A Brief Review. Nat. Prod. Commun. 2 (12), 1297-1302 (2007).
- Tajkarimi, M. M., Ibrahim, S. A., Cliver, D. O. Antimicrobial herb and spice compounds in food. Food Control. 21 (9), 1199-1218 (2010).
- Nedorostova, L., Kloucek, P., Kokoska, L., Stolcova, M., Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control. 20 (2), 157-160 (2009).
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods - A review. Int. J. Food Microbiol. 94 (3), 223-253 (2004).
- Ramanathan, L., Das, N. Studies on the control of lipid oxidation in ground fish by some polyphenolic natural products. J. Agric. Food Chem. 40 (1), 17-21 (1992).
- Yamazaki, K., Yamamoto, T., Kawai, Y., Inoue, N. Enhancement of antilisterial activity of essential oil constituents by nisin and diglycerol fatty acid ester. Food Microbiol. 21 (3), 283-289 (2004).
- García-Díez, J., Alheiro, J., Falco, V., Fraqueza, M. J., Patarata, L. Synergistic activity of essential oils from herbs and spices used on meat products against food borne pathogens. Nat. Prod. Commun. 12 (2), 281-286 (2017).
- Hussein Hamdy Roby, M., Atef Sarhan, M., Abdel-Hamed Selim, K., Ibrahim Khalel, K. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 43, 827-831 (2013).
- Gouveia, A. R., et al. The Antimicrobial Effect of Essential Oils Against Listeria monocytogenes in Sous vide Cook-Chill Beef during Storage. J. Food Process. Preserv. 41 (4), (2017).
- Chen, C., Nace, G., Irwin, P. A 6 x 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods. 55 (2), 475-479 (2003).
- Herigstad, B., Hamilton, M., Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods. 44 (2), 121-129 (2001).
- . Practical food microbiology Available from: https://drive.google.com/file/d/0BzyVOLllJ0B1YmlEemZ5M1RZekU/view?ts=590d8019 (2003)
- Smith-Palmer, A., Stewart, J., Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 26 (2), 118-122 (1998).
- Burt, S. a., Reinders, R. D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 36 (3), 162-167 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved