A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Stiffness Measurement of Soft Silicone Substrates for Mechanobiology Studies Using a Widefield Fluorescence Microscope
In This Article
Summary
Substrates with stiffness in the kilopascal-range are useful to study the response of cells to physiologically relevant micro-environment stiffness. Using just a widefield fluorescence microscope, the Young's modulus of soft silicone gels can be determined using an indentation with a suitable sphere.
Abstract
Soft tissues in the human body typically have stiffness in the kilopascal (kPa) range. Accordingly, silicone and hydrogel flexible substrates have been proven to be useful substrates for culturing cells in a physical microenvironment that partially mimics in vivo conditions. Here, we present a simple protocol for characterizing the Young's moduli of isotropic linear elastic substrates typically used for mechanobiology studies. The protocol consists of preparing a soft silicone substrate on a Petri dish or stiff silicone, coating the top surface of the silicone substrate with fluorescent beads, using a millimeter-scale sphere to indent the top surface (by gravity), imaging the fluorescent beads on the indented silicone surface using a fluorescence microscope, and analyzing the resultant images to calculate the Young's modulus of the silicone substrate. Coupling the substrate's top surface with a moduli extracellular matrix protein (in addition to the fluorescent beads) allows the silicone substrate to be readily used for cell plating and subsequent studies using traction force microscopy experiments. The use of stiff silicone, instead of a Petri dish, as the base of the soft silicone, enables the use of mechanobiology studies involving external stretch. A specific advantage of this protocol is that a widefield fluorescence microscope, which is commonly available in many labs, is the major equipment necessary for this procedure. We demonstrate this protocol by measuring the Young's modulus of soft silicone substrates of different elastic moduli.
Introduction
Cells in soft tissues reside in a micro-environment whose stiffness is in the kilopascal range1, in contrast to tissue culture dishes whose stiffness is several orders of magnitude higher. Early experiments with cells on extracellular matrix protein-coated soft substrates showed that the substrate stiffness influences how cells move on as well as adhere to the extracellular matrix beneath2,3. In fact, the substrate stiffness fundamentally influences the cell function4 in a manner similar to pervasive biochemical signals. Polyacrylamide gels (coated with extracellular matrix proteins) are (water-permeating) hydrogels that have been extensively used as cell culture substrates for mechanobiology studies5. Polydimethylsiloxane (PDMS), the most common silicone (polysiloxane), has been widely used as a stiff silicone with megapascal-range stiffness for micron-scale fabrication6. More recently, soft silicone substrates with stiffness in the more physiologically relevant kilopascal range have been employed as cell culture substrates for mechanobiology studies7,8.
Several methods have been used to measure the stiffness of flexible substrates, including atomic force microscopy, macroscopic deformation of whole samples upon stretching, rheology, and indentation using spheres and spherically tipped microindentors9. While each technique has its own advantages and disadvantages, indentation with a sphere is an especially simple yet fairly accurate method that only requires the access to a widefield fluorescence microscope. Indentation with a metallic sphere has been used to measure the stiffness of hydrogels in prior work3,9,10. Early work that demonstrated the importance of substrate stiffness to cell movement utilized this method to determine hydrogel substrate stiffness3. More recently, confocal microscopy has also been used for an elegant characterization10.
Here, we present a step-by-step protocol for preparing a soft silicone substrate, coupling fluorescent beads (and an extracellular matrix protein such as collagen I) just to the top surface, imaging an indenting sphere and the top surface using phase and fluorescence imaging, respectively, and finally analyzing the images to compute the Young's modulus of the silicone substrate. The soft silicone substrate prepared in this manner can be readily used for traction force microscopy experiments. The use of stiff silicone (instead of a Petri dish) as the base for the soft silicone also enables mechanobiology studies using an external stretch. Where warranted, practical considerations necessary for avoiding possible complications are also indicated.
Protocol
1. Fabrication of Soft Silicone Substrate
- Weigh out 1.75 g of the A component and 1.75 g of the B component (A:B = 1:1) from the soft silicone elastomer kit using (polystyrene) weighing trays.
- Add the A component to the B component in the weighing tray and mix them together for 5 min using an appropriate applicator stick.
- Add the above mixture to a 35 mm Petri dish. Allow the mixture to spread evenly across the Petri dish for a couple of minutes.
Note: The choice of the Petri dish diameter and the amount of soft silicone will determine the soft silicone thickness. Here, the thickness will be around 3.5 mm; more about choosing the elastomer thickness in the Discussion section. - Place the Petri dish with the silicone mixture, with the lid off, in a vacuum chamber for 15 min to remove any air bubbles. During this time, pre-heat a hot plate to 70 °C.
- Once the hot plate reaches 70 °C, place a glass slide on it and then place the Petri dish with the silicone mixture on the glass slide. Let the silicone cure at 70 °C for 30 min. Do not place the polystyrene dish directly on the hot plate, as the Petri dish may melt.
2. Coupling of Fluorescent Microbeads to the Soft Silicone
- Place the cured soft silicone (in the uncovered Petri dish) in a deep UV chamber (an enclosure with a deep UV lamp of light wavelengths of 185 and 254 nm). Expose the soft silicone sample (~5 - 10 cm away from the UV lamp) to deep UV light for 5 min.
- While the silicone is being exposed to deep UV light, proceed with steps 2.2 - 2.6 below. After the deep UV exposure, degas the deep UV chamber for at least 5 min before retrieving the sample.
- In the meantime, weigh out 19 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) in a 1.5 mL microcentrifuge tube and add 500 μL of deionized water (DI) water to it. Dissolve the EDC by gently shaking the tube.
- In a separate 1.5 mL microcentrifuge tube, weigh out 11 mg of N-hydroxysulfosuccinimide (sulfo-NHS), add 500 μL of DI water to it, and dissolve the sulfo-NHS by gently shaking the tube. Then, combine the EDC and sulfo-NHS solutions in a single microcentrifuge tube.
- To this EDC/sulfo-NHS solution, add 30 μL of 0.44 μm diameter red (or any other fluorescence color based on the filter cubes available in the fluorescence microscope) carboxylate modified fluorescent microbeads (with a 1% w/v stock concentration).
- To the EDC/sulfo-NHS/bead mixture, add 0.02 mg of collagen I (from a rat tail, stock concentration of 4 mg/mL in 0.02 M acetic acid) to obtain a concentration of about 0.02 mg/mL.
- Vortex the EDC/NHS/bead/collagen I mixture briefly to ensure that the beads are evenly dispersed throughout, before coupling.
- Pipette 1 mL of the EDC/NHS/bead/collagen I mixture on a piece of parafilm placed on top of another shallow, flat lid (of smaller diameter). Invert the Petri dish with soft silicone on this mixture so that the soft silicone surface contacts the mixture but does not directly touch the surface of the smaller Petri dish lid below. To raise the inverted Petri dish, use one or two glass slides under either side of the inverted Petri dish spacers.
Note: Please refer to Figure 1 to see how step 2.7 is performed. - Cover the sample with aluminum foil and incubate it at room temperature for 30 min.
- Remove the Petri dish with soft silicone and set it upright (silicone-side up).
- Wash the soft silicone surface with phosphate-buffered saline (PBS) by adding 2 mL of PBS (pH 7.4) to the dish. Let it sit for a couple of minutes. Aspirate off the PBS and wash the silicone again with 2 mL of PBS. Let the silicone cure further for about a day. For that reason, place the soft silicone sample in PBS at 37 °C overnight.
3. Measurement of Silicone Stiffness with Sphere Indentation using a Widefield Fluorescence Microscope
- Retrieve the Petri dish with soft silicone and ensure that it contains at least 1 mL of PBS to have the silicone surface several mm below the liquid surface.
- Using pointed tweezers, drop five 1 mm zirconium sphere indentors on the soft silicone. Immerse the spheres into the liquid medium and drop them, away from the edges of the silicone layer and at least 5 indentor diameters away from the location of the other indentors.
Note: When dropped above the liquid surface, the spheres may fail to enter the liquid medium (float) due to the liquid medium’s surface tension. - Place the Petri dish with soft silicone on the microscope stage so that it is possible to image through the Petri dish base.
- Using phase imaging with a 10X objective (such as a dry 10X objective of NA 0.30), locate and bring a sphere indentor into focus.
- Take a phase image of a part or the whole of the indentor and save this image. Use a tile scan if available. If the indentor has any visible defects, discard and replace it with another indentor.
- Under live phase imaging, pan to the left of the indentor’s edge so that the left edge of the frame is at least a distance of ~1.5 R from the indentor center. Ensure that the center of the indentor remains visible on the right side, close to the right edge of the image frame. Take a phase image and save it.
- Switch the microscope light source to the illumination for the red fluorescent channel. With the x- and y-coordinates unchanged (the x-y position of the indentor center within but near the right edge of the frame), focus down (decrease Z) until the red fluorescent microbeads under the sphere indentor’s center just go out of focus.
- Take a z-stack with an image for every z-increment of 0.5 µm till the microbeads in the top layer of the silicone far from the indentor (near the left edge of the imaging frame) go out of focus.
- Repeat steps 3.4 - 3.8 with the other indentors on the sample.
4. Calculating the Silicone’s Stiffness (Young’s Modulus)
- Open the phase image of the indentor using ImageJ, click on the line tool, and measure the indentor’s diameter in pixels. Click and hold on a point on the indentor edge, move the cursor to a diametrically opposite point on the edge and note the length in pixels displayed on the status bar of the ImageJ main window before releasing the cursor.
- Ensure that the unit of length is set to pixels by clicking Analyze | Set Scale and checking the Unit of length.
- Convert the indentor’s radius in pixels to μm by taking into account the objective magnification and the CCD camera pixel size (R in μm = R in pixels x the CCD camera pixel size in μm / the objective magnification).
- Open the red channel z-stack of microbead images (if the microbeads are red fluorescent) in ImageJ by clicking on File | Import | Image Sequence and select any image in the stack and click OK to open the stack.
Note: F1 is the frame number at which the microbeads under the indentor center are in the best possible focus and F2 is the frame number at which the microbeads (at a region of ~1.5 R away from the bead center) near the left edge of the frame are in the best possible focus. The z-difference between the two frames is the indentation depth δ.- Using the line tool in ImageJ, draw a line across a well-defined microbead in the image. Click on Analyze | Plot Profile and click on the Live button to obtain the updated line scan intensity across the bead while selecting different frames. The frame that gives the highest value of the maximum intensity can be chosen as the frame in focus.
- Since the z-increment between the frames in the z-stack is 0.5 μm, calculate the indentation depth in μm as δ = (F2-F1) x 0.5.
- Calculate the force exerted on the gel by the indentor due to its weight (minus the opposing buoyant force), that is, the indentation force F, as the volume of the indentor x (the density of the indentor - the density of the liquid medium) x the acceleration due to gravity. Use the equation F = (4/3) x 3.142 x (R3) x (ρindentor - ρmedium) x g where R is the radius of the indentor, ρindentor is the density of the indentor, ρmedium is the density of the liquid medium and g is the acceleration due to gravity (9.81 m/s2). Express all quantities on the right-hand side in SI units to obtain F (in N).
- Calculate the Young’s modulus (E) of the silicone using a modified11 Hertz model12 equation:
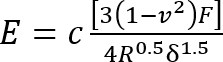
Where:
c = a correction factor that modifies the Hertz model expression that follows it;
v = Poisson’s ratio of the silicone gel (taken as 0.5 as for incompressible materials7);
F = the indentation force;
R = the indentor radius; and
δ = the indentation depth.
Express all quantities on the right-hand side in SI units to obtain E in Pa.- Calculate the correction factor c as follows3:
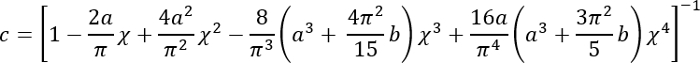
Where:
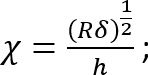
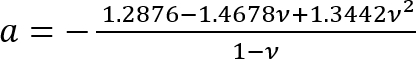 ; and
; and
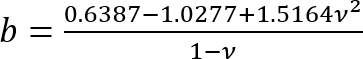 .
.
It should be noted that this correction factor is specifically to be used only when the soft silicone adheres well to the Petri dish (or stiff silicone) underneath it (which is the case here). - Calculate the height h of the soft silicone layer based on the amount of silicone added and the Petri dish diameter. Alternatively, obtain h directly by determining the z-coordinate of the top and bottom surfaces of the silicone layer by phase imaging (minor impurities come into focus at either surface). Note that for a large h (h2 > Rδ), the correction factor c is close to 1.
- Calculate the correction factor c as follows3:
- Repeat steps 4.1 - 4.4 for each indentor. Average the Young’s modulus obtained from each indentor to obtain the mean Young’s modulus for the silicone sample.
Results
Using the protocol detailed above, we prepared soft silicone in a 35 mm Petri dish, cured it at 70 °C for 30 min and coupled fluorescent microbeads (and collagen I) to the top surface as schematically depicted in Figure 1. Deep UV has been used previously for the eventual protein coupling to substrates13. Note that (I) the curing conditions used here are specific to this soft silicone and (II) the indentation measurement is pe...
Discussion
While the sphere indentation method is easy to implement, careful attention must be paid to the choice of indentor and the thickness of the soft silicone sample. The equation used to calculate the Young's modulus is valid under a set of conditions11and these are typically satisfied when the thickness of the silicone sample is > 10% of the indentor radius and < ~13x the indentor radius. We found that a silicone thickness of 5 - 10x the indentor radius was a good choice, wherein the samp...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Margaret Gardel for generously allowing the use of the rheometer. We acknowledge support from the NIH (1R15GM116082) that enabled this work.
Materials
| Name | Company | Catalog Number | Comments |
| CY 52-276 A/B silicone elastomer kit | Dow Corning | CY 52-276 | Store at room temperature |
| Thermo Scientific Pierce EDC | Fisher Scientific | PI22980 | Store at -20°C |
| Thermo Scientific Pierce Sulfo-NHS crosslinker | Fisher Scientific | PI-24510 | Store at 4°C |
| Carboxyl fluorescent pink particles, 0.4-0.6 µm, 2 mL | Spherotech, Inc. | CFP-0558-2 | Store at 4°C, do not freeze |
| 1.0 mm Acid washed Zirconium beads | OPS Diagnostics LLC | BAWZ 1000-250-33 | |
| Deep UV chamber with ozone evacuator | Novascan Technologies, Inc. | PSD-UV4, OES-1000D | |
| Wide field fluorescence microscope | Leica Microsystems | DMi8 | |
| Collagen I, from rat tail | Corning | 354236 | Stock concentration = 4 mg/ml; store at 4°C |
| ImageJ-NIH | N/A | N/A | public-domain software |
References
- Handorf, A. M., Zhou, Y., Halanski, M. A., Li, W. J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 11 (1), 1-15 (2015).
- Pelham, R. J., Wang, Y. -. L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences. 94 (25), 13661-13665 (1997).
- Lo, C. M., Wang, H. B., Dembo, M., Wang, Y. L. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 79, 144-152 (2000).
- Discher, D. E., Janmey, P., Wang, Y. -. L. Tissue cells feel and respond to the stiffness of their Substrate. Science. 310, 1139-1143 (2005).
- Kandow, C. E., Georges, P. C., Janmey, P. A., Beningo, K. A. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods in Cell Biology. 83, 29-46 (2007).
- Johnston, I. D., McCluskey, D. K., Tan, C. K. L., Tracey, M. C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. Journal of Micromechanics and Microengineering. 24 (3), 035017 (2014).
- Style, R. W., et al. Traction force microscopy in physics and biology. Soft Matter. 10 (23), 4047-4055 (2014).
- Lee, E., et al. Deletion of the cytoplasmic domain of N-cadherin reduces, but does not eliminate, traction force-transmission. Biochemical and Biophysical Research Communications. 478 (4), 1640-1646 (2016).
- Frey, M. T., Engler, A., Discher, D. E., Lee, J., Wang, Y. L. Microscopic methods for measuring the elasticity of gel substrates for cell culture: microspheres, microindenters, and atomic force microscopy. Methods Cell Biol. 83, 47-65 (2007).
- Lee, D., Rahman, M. M., Zhou, Y., Ryu, S. Three-dimensional confocal microscopy indentation method for hydrogel elasticity measurement. Langmuir. 31 (35), 9684-9693 (2015).
- Dimitriadis, E. K., Horkay, F., Maresca, J., Kachar, B., Chadwick, R. S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophysical Journal. 82 (5), 2798-2810 (2002).
- Hertz, H. Über die Berührung fester elastischer Körper. Journal für die reine und angewandte Mathematik. 92, 156-171 (1882).
- Azioune, A., Carpi, N., Tseng, Q., Théry, M., Piel, M., Cassimeris, L., Tran, P. Protein micropatterns: a direct printing protocol using deep UVs. Microtubules: In Vivo. , 133-146 (2010).
- Bashirzadeh, Y., Qian, S., Maruthamuthu, V. Non-intrusive measurement of wall shear stress in flow channels. Sensors and Actuators A: Physical. 271, 118-123 (2018).
- Muhamed, I., Chowdhury, F., Maruthamuthu, V. Biophysical tools to study cellular mechanotransduction. Bioengineering (Basel). 4 (1), 12 (2017).
- Dumbali, S. P., Mei, L., Qian, S., Maruthamuthu, V. Endogenous sheet-averaged tension within a large epithelial cell colony. Journal of Biomechanical Engineering. 139 (10), 101008 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved