A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Quantitative Analysis by Thermogravimetry-Mass Spectrum Analysis for Reactions with Evolved Gases
In This Article
Summary
Precise determination of the evolved gases' flow rate is key to study the details of reactions. We provide a novel quantitative analysis method of equivalent characteristic spectrum analysis for thermogravimetry-mass spectrum analysis by establishing the calibration system of the characteristic spectrum and relative sensitivity, for obtaining the flow rate.
Abstract
During energy conversion, material production, and metallurgy processes, reactions often have the features of unsteadiness, multistep, and multi-intermediates. Thermogravimetry-mass spectrum (TG-MS) is seen as a powerful tool to study reaction features. However, reaction details and reaction mechanics have not been effectively obtained directly from the ion current of TG-MS. Here, we provide a method of an equivalent characteristic spectrum analysis (ECSA) for analyzing the mass spectrum and giving the mass flow rate of reaction gases as precise as possible. The ECSA can effectively separate overlapping ion peaks and then eliminate the mass discrimination and temperature-dependent effect. Two example experiments are presented: (1) the decomposition of CaCO3 with evolved gas of CO2 and the decomposition of hydromagnesite with evolved gas of CO2 and H2O, to evaluate the ECSA on single-component system measurement and (2) the thermal pyrolysis of Zhundong coal with evolved gases of inorganic gases CO, H2, and CO2, and organic gases C2H4, C2H6, C3H8, C6H14, etc., to evaluate the ECSA on multi-component system measurement. Based on the successful calibration of the characteristic spectrum and relative sensitivity of specific gas and the ECSA on mass spectrum, we demonstrate that the ECSA accurately gives the mass flow rates of each evolved gas, including organic or inorganic gases, for not only single but multi-component reactions, which cannot be implemented by the traditional measurements.
Introduction
Understanding in depth the real features of a reaction process is one critical issue for the development of advanced materials and the establishment of a new energy conversion system or metallurgy production process1. Almost all reactions are carried out under unsteady conditions, and because their parameters, including the concentration and flow rate of reactants and products, always change with the temperature or pressure, it is difficult to clearly characterize the reaction feature by only one parameter, for instance through the Arrhenius Equation. In fact, the concentration implies only the relationship between the component and the mixture. Real reaction behavior might not be affected, even though the concentration of a component in one complicated reaction is adjusted to a great extent since the other components might have a stronger influence on it. On the contrary, the flow rate of each component, as an absolute quantity, can give persuasive information to understand the characteristics of the reactions, especially very complicated ones.
At present, the TG-MS coupling system equipped with the electron ionization (EI) technique has been used as a prevalent tool for analyzing the features of reactions with evolved gases2,3,4. However, first, it should be noted that the ion current (IC) obtained from an MS system makes it difficult to directly reflect the flow rate or concentration of the evolved gas. The massive IC overlap, fragment, severe mass discrimination, and diffusion effect of gases in the furnace of a thermogravimeter can greatly hamper the quantitative analysis for TG-MS5. Second, EI is the most common and readily available strong ionization technique. An MS system equipped with EI easily results in fragments and does not often directly reflect some organic gases with a larger molecular weight. Therefore, MS systems with different soft ionization techniques (e.g., photoionization [PI]) are required simultaneously to be hyphenated to a thermobalance and applied to evolved gas analysis6. Third, the intensity of the IC at some mass-to-charge ratios (m/z) cannot be used to determine the dynamic characteristic of any reaction gas, because it is often affected by the other ICs for a complex reaction with multicomponent evolved gases. For example, the drop in the IC curve of a specific gas does not necessarily indicate a decrease in its flow rate or concentration; instead, maybe it is affected by the other gases in the complex system. Thus, it is important to take into account all gases' ICs, certainly with a carrier gas and inert gas.
In fact, quantitative analysis based on mass spectrum greatly depends on the determination of the calibration factor and relative sensitivity of the TG-MS system. Maciejewski and Baiker7 investigated in a thermal analyzer-mass spectrometer (TA-MS) system, in which the TA is connected by a heated capillary to a quadrupole MS, the effect of experimental parameters, including the concentration of gases species, temperature, flow rate, and properties of the carrier gas, on the sensitivity of the mass spectrometric analysis. The evolved gases were calibrated by the decomposition of the solids via a well-known, stoichiometric reaction and injecting a certain amount of gas into the carrier gas stream with a constant rate. The experimental results show that there is a negative linear correlation of the MS signal intensity of evolved gas to that of the carrier gas flow rate, and the evolved gas MS intensity is not influenced by the temperature and the amount of analyzed gas. Further, based on the calibration method, Maciejewski et al.8 invented the pulse thermal analysis (PTA) method, which provides an opportunity to determine the flow rate by simultaneously monitoring the changes of the mass, enthalpy, and gas composition resulted from the reaction course. However, it is still hard to give persuasive information about the complicated reaction (e.g., coal combustion/gasification) by using the traditional TG-MS analysis or PTA methods.
In order to overcome the difficulties and disadvantages of the traditional measuring and analysis method for the TG-MS system, we developed the quantitative analysis method of ECSA9. The fundamental principle of ECSA is based on the TG-MS coupling mechanism. The ECSA can take into account all gases' ICs, including the reaction gases', carrier gases', and inert gases'. After building the calibration factor and relative sensitivity of some gas, the real mass or molar flow rate of each component can be determined by the calculation of the IC matrix (i.e., the mass spectrum of TG-MS). Compared with the other methods, ECSA for the TG-MS system can effectively separate the overlapping spectrum and eliminate the mass discrimination and the temperature-dependent effect of TG. The data produced by ECSA have proved to be reliable via a comparison between the mass flow rate of evolved gas and mass loss data by differential thermogravimetry (DTG). In this study, we used an advanced TG-DTA-EI/PI-MS instrument10 to carry out the experiments (Figure 1). This instrument consists of a cylindrical quadrupole MS and a horizontal thermogravimetry-differential thermal analyzer (TG-DTA) equipped with both EI and PI mode, and with a skimmer interface. ECSA for the TG-MS system determines the physics parameters of all evolved gases by utilizing the actual TG-MS coupling mechanism (i.e., an equal relative pressure) to implement the quantitative analysis. The overall analysis process includes a calibration, the test itself, and data analysis (Figure 2). We present two example experiments: (1) the decomposition of CaCO3 with only evolved gas of CO2 and the decomposition of hydromagnesite with evolved gas of CO2 and H2O, to evaluate the ECSA on a single-component system measurement and (2) the thermal pyrolysis of brown coal with evolved gases of inorganic gases CO, H2, and CO2, and organic gases CH4, C2H4, C2H6, C3H8, C6H14, etc., to evaluate the ECSA on a multi-component system measurement. ECSA based on the TG-MS system is a comprehensive solution method for quantitatively determining the amount of evolved gas in thermal reactions.
Protocol
1. Calibration of ECSA for the TG-MS System
- Calibration of the characteristic spectrum
- Prepare the evolved gases of CO2, H2O, CH4, He, etc. to be calibrated, modulating the gas pressure at 0.15 MPa.
- Connect the gas cylinder to the TG-MS system by stainless steel tube and purge the individual gas into the TG-MS system with a flow rate of 100 mL/min.
- Monitor the mass spectrum of the individual gas. Carefully watch and compare the characteristic peak of gases to be calibrated and the possible impurity gases in the mass spectrum by TG-MS to confirm the species and purity of the gases.
NOTE: The above-mentioned gases can be bought directly in gas cylinders or decomposed from some testing samples (except He). He is used as carrier gas in both the calibration and the test.
CAUTION: For some substances which are harmful to the TG or MS, the carrier gas must be used.
- Calibration of the relative sensitivity
- Purge the reference gas He with a flow rate of 300 mL/min into the TG-MS system for 20 min to clean the system.
- Purge synchronously one type of calibrated gas, such as CO2 or H2O, and the reference gas He into the TG-MS system with a flow rate of 100 mL/min.
- Calculate the relative sensitivity of each gas according to the known flow rate and the mass spectrum (Equation 1).

Here,
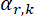 = relative sensitivity of the k gas to the reference gas
= relative sensitivity of the k gas to the reference gas
 = the given flow rate of the reference gas
= the given flow rate of the reference gas
 = the given flow rate of the k gas
= the given flow rate of the k gas
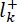 = the determined ion current for the k gas by MS, and
= the determined ion current for the k gas by MS, and
 = the determined ion current for reference gas.
= the determined ion current for reference gas.
NOTE: The volumetric flow rates of the calibrated and reference gas must be known in advance.
2. Testing Process of ECSA for the TG-MS System
- Preparation of the samples used for the test
- Preparation of the samples of CaCO3 and hydromagnesite
- Collect 10-g samples of CaCO3 with an average diameter of 15 µm.
- Collect 10 g of a white block of hydromagnesite, break it into pieces of < 3 mm in size, and grind the pieces with a machine-stirred mill to approximately 10 µm.
- Dry all the samples for 24 h in the oven at a temperature of 105 °C.
NOTE: The above steps can be implemented in parallel.
- Preparation of the samples of Zhundong coals
- Collect 20 g of Zhundong coal from the coalfield located at the Mori Kazak Autonomous County, Xinjiang province in China.
- To eliminate any external moisture, dry the coal in the oven at a temperature of 105 °C for 24 h.
- Break and ground the coal in a mill to obtain a particle size range of 180 - 355 m.
- Preparation of the samples of CaCO3 and hydromagnesite
- Test of the thermal reactions
- Purge the TG-MS system with the carrier gas He for 2 h to expel the air and moisture. Meanwhile, preheat the instrument to around 500 °C and, then, cool it down to room temperature.
NOTE: The He gas was used as carrier gas for all the tests. - Monitor the atmosphere by using MS in the first 20 min, carefully watching and comparing the characteristic peak of CO2, He, and the impurity gases of O2, N2, and H2O in the mass spectrum, to guarantee the lowest content of air and moisture, not affecting the experimental measurements.
- Weigh a sample of 10 mg by using the precision electronic balance and put the sample into an Al2O3 crucible.
- Put the Al2O3 crucible with the sample into the TG and close the furnace.
- Set the operating parameters. (1) For the CaCO3 test, start the temperature at 20 °C and heat to 550 °C with a heating rate of 10 K/min; then, for the modulating temperature program, heat to 800 °C with alternating heating rates of 10 K/min and 20 K/min. (2) For the hydromagnesite and coal test, start the temperature at 20 °C and use a heating rate of 10 K/min, a hold time of 15 min, a stopping temperature of 1,000 °C, and a gas flow rate of 20 mL/min; keep an m/z range of 2 - 200 for mode EI and 10 - 410 for mode PI.
NOTE: Mode PI was used to identify the organic gases, mainly used for the test of Zhundong coal pyrolysis in this study.
- Purge the TG-MS system with the carrier gas He for 2 h to expel the air and moisture. Meanwhile, preheat the instrument to around 500 °C and, then, cool it down to room temperature.
3. Qualitative and Quantitative Analysis
- Get the 3-D mass spectrum data recorded by the computer connected with the TG-MS instrument.
- Calculate the actual parameters, including the mass flow rate and the concentration of each evolved gas, by using the ECSA method, based on the determined calibrated characteristic peak (step 2.1) and the relative sensitivity (step 2.2).
- Analyze the thermal reaction according to the actual parameters9.
Results
The thermal decomposition of CaCO3 is a relatively simple reaction, which was used to demonstrate the applicability of the ECSA method. After calibrating the characteristic peak and relative sensitivity of CO2 to carrier gas He, the actual mass flow rate of CO2 evolved by the thermal decomposition of CaCO3 was calculated by the ECSA method and was compared with the actual mass loss (Figure 3). It is shown that there...
Discussion
This protocol could be easily modified to accommodate other measurements for studying evolved gases and pyrolysis reactions by a TG-MS system. As we know, the evolved volatile from the pyrolysis of biomass, coal, or other solid/liquid fuel does not always include only the inorganic gases (e.g., CO, H2, and CO2) but also the organic ones (e.g., C2H4, C6H5OH, and C7H8). Moreover, massive fragments would result from the...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51506199).
Materials
| Name | Company | Catalog Number | Comments |
| CaCO3 and Ca(OH)2 | Sinopharm Chemical Reagent | ||
| hydromagnesite | Bangko Coarea in Tibet | ||
| Zhundong coal | the coal field in the Mori Kazak Autonomous County, Junggar basin, Xinjiang province of China | ||
| ThermoMass Photo/H | Rigaku Corporation | ||
| The STA449F3 synchronous thermal analyzer and QMS403C quadrupole MS analyzer | NETZSCH |
References
- Li, R. B., Xia, H. D., Wei, K. . 15th International Conference on Clean Energy (ICCE-2017). , (2017).
- Zou, C., Ma, C., Zhao, J., Shi, R., Li, X. Characterization and non-isothermal kinetics of Shenmu bituminous coal devolatilization by TG-MS. Journal of Analytical and Applied Pyrolysis. 127, 309-320 (2017).
- Jayaraman, K., Kok, M. V., Gokalp, I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renewable Energy. 101, 293-300 (2017).
- Tsugoshi, T., et al. Evolved gas analysis-mass spectrometry using skimmer interface and ion attachment mass spectrometry. Journal of Thermal Analysis and Calorimetry. 80 (3), 787-789 (2005).
- JaenickeRossler, K., Leitner, G. TA-MS for high temperature materials. Thermochimica Acta. (1-2), 133-145 (1997).
- Fendt, A., Geissler, R., Streibel, T., Sklorz, M., Zimmermann, R. Hyphenation of two simultaneously employed soft photo ionization mass spectrometers with thermal analysis of biomass and biochar. Thermochimica Acta. , 155-163 (2013).
- Maciejewski, M., Baiker, A. Quantitative calibration of mass spectrometric signals measured in coupled TA-MS system. Thermochimica Acta. 295 (1-2), 95-105 (1997).
- Maciejewski, M., Muller, C. A., Tschan, R., Emmerich, W. D., Baiker, A. Novel pulse thermal analysis method and its potential for investigating gas-solid reactions. Thermochimica Acta. 295 (1-2), 167-182 (1997).
- Xia, H. D., Wei, K. Equivalent characteristic spectrum analysis in TG-MS system. Thermochimica Acta. 602, 15-21 (2015).
- Li, R. B., Chen, Q., Xia, H. D. Study on pyrolysis characteristics of pretreated high-sodium (Na) Zhundong coal by skimmer-type interfaced TG-DTA-EI/PI-MS system. Fuel Processing Technology. 170, 79-87 (2018).
- Li, C. Z. Some recent advances in the understanding of the pyrolysis and gasification behaviour of Victorian brown coal. Fuel. 86 (12-13), 1664-1683 (2007).
- Song, H. J., Liu, G. R., Zhang, J. Z., Wu, J. H. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Processing Technology. 156, 454-460 (2017).
- Kashimura, N., Hayashi, J., Li, C. Z., Sathe, C., Chiba, T. Evidence of poly-condensed aromatic rings in a Victorian brown coal. Fuel. 83 (1), 97-107 (2004).
- Li, C. Z., Sathe, C., Kershaw, J. R., Pang, Y. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Fuel. 79 (3-4), 427-438 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved