Method Article
Detection of 3-Nitrotyrosine in Atmospheric Environments via a High-performance Liquid Chromatography-electrochemical Detector System
In This Article
Summary
We present a method to detect 3-nitrotyrosine chemical modifications of atmospheric proteins with 6 mm-diameter rounds cut from air sampler prefilters using a high-performance liquid chromatography-electrochemical detector (HPLC-ECD).
Abstract
3-nitrotyrosine (3-NT) is generated from the tyrosine residue in atmospheric bio-aerosol proteins via a reaction with ozone (O3) and nitrogen dioxide (NO2). Stable 3-NT is a specific marker for oxidative damage and is reported to have a promotive effect to elicit allergies. In the present study, we report the development of a highly sensitive assay to quantify 3-NT in air sampler filters to collect < 2.5 µm of particulate matter (PM2.5) from urban environmental air, including bio-aerosol. In this method, a 6 mm-diameter round hole was cut from the filters of air samplers and mixed with a nonspecific protease cocktail in order to hydrolyze proteins. Protein samples digested to the amino acid level were tested for 3-NT using a high-performance liquid chromatography-electrochemical detector (HPLC-ECD). The maximum 3-NT content was detected in a prefilter for PM of sizes from 4.5 to 7.3 μm, with a detection limit of 1.13 pg/m3.
Introduction
Aerosol or airborne PM contains proteins from various biological origins, including viruses, prokaryotes, fungi, plants (pollen), and animals (insects, human)1. The ratio of protein content in PM is estimated to be nearly 5%, which has been implicated to play a major role as an airborne allergen2. Recent reports suggest that urban ambient aerosol of various sizes contain amino acids with ratios ranging between 0.5% and 2%3. In addition, chemical modifications of PM proteins have been suggested to be generated by the reactions of proteins with various pollutants, such as O3, nitrogen dioxide (NO2), and sulfur dioxide (SO2)4.
3-NT—a protein modification—is generated by the nitration of tyrosine residues. A higher concentration of O3 and NO2 has been shown to promote the nitration of protein molecules in pseudo-atmospheric space5,6. The nitration of tyrosine residues in the polypeptide chain enhances the allergenic potential of pollen proteins7.Thus, the quantification and assessment of airborne 3-NT is an important aspect in addressing the concerns of environmental health.
3-NT is also known as a biomarker of oxidative and nitrosative stress8. An emerging body of evidence has shown a significant association of 3-NT content with several human diseases9,10. Owing to its detrimental effects, the detection and quantitative estimation of 3-NT in a biological sample hold great relevance in determining the condition of an individual's health. In recent years, diverse methods have been introduced to estimate the 3-NT content, including enzyme-linked immunosorbent assays, HPLC, and LC/MS11. In previous studies, we have reported a detection method using the HPLC-ECD technique bestowed with several advantages compared to previous methods. For example, the HPLC-ECD does not require an extraction or derivatization procedure which makes it a relatively simple assay system12.We have confirmed that this method is applicable to detect 3-NT from biological samples (plasma from human and rat).
Although many investigations have emphasized the detection of 3-NT in biological samples, its detection in nonbiological samples remains elusive, and hence the present study has been pursued. In fact, to assess the harmful effect of airborne 3-NT, a quantitative method that is useful to detect 3-NT directly from airborne particles is required; therefore, we applied the existing HPLC-ECD method to detect 3-NT from PM2.5 collected on a glass-fiber filter. By using the technique, 3-NT could be detected directly from small pieces (6 mm-diameter round holes) of the sample from a PM collection filter.We further evaluated the content of 3-NT for various PM sizes and measured the detection limit of 3-NT.The present article proposes a high-sensitive and high-throughput method to detect 3-NT directly from both nonbiological and biological samples.
Protocol
1. Collection of Total Suspended Particles, PM2.5, or PM7, and the Hydrolysis Method for PM Proteins
- Collect the PM from a prefilter or backup filter.
- Prior to the collection of total suspended particles (TSP), PM2.5, and PM7, weigh the quartz filter.
Note: Given that contamination by PM proteins and peptides should not affect the detection of tyrosine nitration (as they lack such a nitro group), we did not treat the quartz filter at a high temperature before the measurement. Additionally, 3-NT content in the quartz filter was under the detection limit, as determined by HPLC-ECD. - Set the quartz filter on a high-volume air sampler with a particle size selector to collect PM7 at a flow rate of 1,000 L/min continuously for 7 d (Figure 1B). TSP can be collected on a quartz filter without a size selector (Figure 1A).
- Collect PM2.5 using a low-volume air sampler with a size classification unit at a flow rate of 116 L/min continuously for 7 d. Using this unit, particles can be classified as PM4.5-2.5, PM7.3-4.5, PM15-7.3, and TSP-PM15 and collected onto quartz filters for classification. PM2.5 can be collected onto a backup filter (Figure 1C).
- Record the total flow volume at the time of the filter collection. Measure the weight of the TSP, PM2.5, and PM7 on the filters by subtracting the precollection weight from the postcollection weight. Seal the filter in a plastic bag and store it at -30 °C until the next step (see section 2).
- Prior to the collection of total suspended particles (TSP), PM2.5, and PM7, weigh the quartz filter.
- Proteolyze the samples.
- Dissolve nonspecific protease cocktail with a 0.1 M acetate buffer (pH 7.4). Put the solution into a dialysis membrane (molecular weight cutoff = 5,000 Da) and immerse it in 1 L of 0.1 M acetate buffer with stirring at 4 °C. Replace the buffer 2x every 12 h, and then perform dialysis overnight in order to remove endogenous 3-NT and nitrite (NO2-). After the dialysis, collect the solution and measure the protein concentration by bicinchoninic acid (BCA) protein assay.
- Punch round circles of 6 mm out of the prefilter or the backup filter and put them into microtubes. Up to five pieces of 6 mm fractions can be used for the analysis (Figure 2).
- Add 300 µL of 0.1 M acetate buffer containing 50 µg of protein of the nonspecific protease cocktail (prepared in step 1.2.1) to the tube to hydrolyze the PM proteins at 50 °C for 16 h with rotation.
Note: To subtract endogenous 3-NT from the nonspecific protease cocktail solution, it is necessary to prepare and digest a nonspecific protease cocktail-only sample. - Rinse the ultrafiltration membrane along with its supplied tube by adding 0.1 M acetate buffer and centrifuging (10,000 x g for 30 sec; the exact speed depends on the membrane), due to contaminating chemicals similar to 3-NT in the membrane. Repeat the rinse 1x.
- Post-proteolysis, centrifuge the samples at 2,500 x g for 10 min. Load the supernatant into the ultrafiltration membrane and centrifuge it at 10,000 x g and 4 °C for ultrafiltration (MWCO = 10,000), until sufficient volume of elution is collected. Store the eluates at 4 °C in the dark.
CAUTION: Due to the detection of a similar peak to 3-NT in the spin column, it is necessary to wash it well with clean water or with a buffer such as HPLC water and a 0.1 M acetate buffer prior to use.
2. Determination of 3-NT Content in TSP, PM2.5, and PM7 via a High-performance Liquid Chromatography and Electrochemical Detection (HPLC-ECD) System
Note: See Figure 3.
- Prepare the mobile phase.
- Prepare 0.2 M phosphate buffer (pH 2.5) containing 50 mg/L EDTA. Measure off 98 volumes of the buffer and two volumes of acetonitrile (HPLC grade), pour them into a clean glass bottle, and mix vigorously with a cap.
- Settle the mobile phase at room temperature for several hours until the dissolved air goes flat.
Note: If the system is started earlier, the mobile phase can be sonicated and degassed under a vacuum. However, over-degassing will change the ratio between the water and organic phase via evaporation.
- The HPLC-ECD system consists of a pump, an HPLC column, a degasser, an ECD, and an injector. Equilibrate the mobile phase and stabilize the system to reduce the background and noise.
- Install the mobile phase and the HPLC column in the HPLC system. Turn on the HPLC pump and stabilize the column with the mobile phase at a flow rate of 500 µL/min at 25 °C column temperature from the day before the start day.
- On the next day, ignite the ECD and set the parameters (the reduction voltage at -900 mV and the excitation voltage at 400 mV). Stabilize it for at least 3 h.
- Measure the 3-NT concentration in the PM7.
- Prepare various concentrations of 3-NT standards (5 - 500 nM) and a blank (without the 3-NT standard and nonspecific protease cocktail, to make sure neither the vial nor the buffer is contaminated) in a 0.1 M acetate buffer.
- Run the HPLC data processor.
- Put sample vials in the auto-injector and set the injection volume to 25 µL. Operate the same conditions of mobile phase and flow rate as mentioned in step 2.2.1.
Note: If the sample preparation is complete, there should be samples, a standard (5 - 500 nM), a nonspecific protease cocktail-only sample, and a blank vial. - Make sure that the baseline in the chromatogram is flat and start the sample injection.
Note: Generally, 3-NT is detected in 20 - 21 min; however, approximately 30 min is recommended as the running time per sample injection. Otherwise, contaminant peaks will be included in the next run.
3. Analysis of Chromatograms and Quantification of 3-NT Content in PM7
- Check the 3-NT peak in the chromatograms and calculate the content.
- After the data collection, open the chromatogram data file with analysis software.
- Draw a line across the 3-NT peak from the flat baseline and read the peak height from the drawn line.
Note: The quantitative limit is approximately 5 nM (125 fmol in a 25 µL injection).
CAUTION: The electrode in the ECD becomes depleted slowly, and the limit will be high due to the S/N ratio. - Prepare a regression line from the 3-NT standard peaks, calculate the slope, and intercept.
- Assign these constants into the following equation and calculate the 3-NT content in the samples as follows.
Sample 3 – NT (nM) = [sample peak – intercept] / slope Eq. (1)
Note: The standard curve should be well represented as a straight line between 5 nM and 500 nM.
- Calculate the 3-NT in the air environment and the PM7 from the sample vial.
- Multiply the 3-NT concentration by the reaction volume (and the dilution factor, if any), and evaluate the absolute 3-NT content in PM7 from the circle.
- Measure the collected filter area and calculate the 3-NT content in the total PM7 filter by the following equation.
Total PM7 = [3-NT content in the PM7 of the circle] x [ratio of the PM7 filter area to the circle area] x 226.19 (in the case of converting moles to grams) Eq. (2) - Evaluate the 3-NT content in the air environment or PM7 by dividing the total PM7 by the total air flow volume or PM7 weight (recorded in step 1.1.3).
Results
The HPLC-ECD principle is simple. Samples containing the target are separated by the HPLC column and mobile phase, and the separated target compound is reduced and/or oxidized in the electrochemical detector.
In the HPLC-ECD system, a 3-NT peak is detected in approximately 22 minutes. ECDs (PEC-510 and HTEC-500) are connected in tandem in the HPLC line. The first ECD reduces the hydroxyl groups in the compounds, and the next oxidizes the reduced chemicals. ECDs have a highly sensitive detection range of 10-15 M. The detection limit of the HPLC-ECD system was 1.13 pg/m3, which was calculated as the mean basal signal (mV) of 10 parts x 3.
Representative chromatograms are shown in Figure 4. By analyzing a pure 3-NT standard, a definitive peak with a stable baseline can be detected (Figure 4A). 3-NT in PM7 was also measured using 6 mm, round-shaped samples (Figure 4B).
Regarding the accuracy of 3-NT detection capability, when a 3-NT standard was injected in HPLC, the peak corresponding to 3-NT was detected at a retention time of 20 minutes. In PM samples, 3-NT was separated from other substances, and the independent peak was detected at the same retention time as the standard. A typical standard curve is shown in Figure 5, where linearity is observed between 5 and 40 nM. Linearity can be seen up to 500 nM (data not shown).
Table 1 shows the 3-NT contents in the atmosphere, calculated as pg/m3 air. The highest concentration of 3-NT in a PM2.5 prefilter was observed in #3 (PM7.3-4.5).
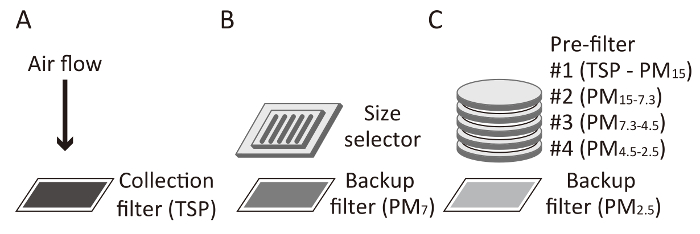
Figure 1: Collection scheme for TSP, PM7, and PM2.5. (A) The total suspended particles (TSP) are collected directly onto a collection filter (at a flow rate of 1,000 L/min). (B) When PM7 is collected, large particles are excluded by a size selector (at a flow rate of 1,000 L/min). (C) PM2.5 is collected through four prefilters (at a flow rate of 116 L/min). A quartz filter is commonly used as a backup filter for PM7 and PM2.5 or as a collection filter for TSP, as shown in this figure. Please click here to view a larger version of this figure.
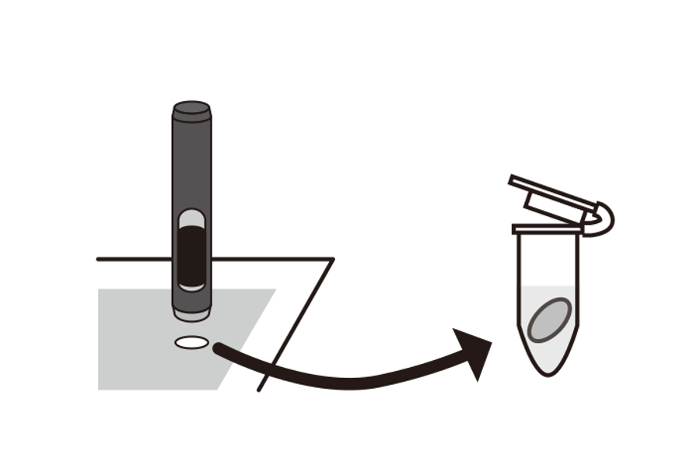
Figure 2: Preparation of a 6 mm-round circle. The collection filter was cut using a hollow punch tool with a 6 mm diameter. Then, the sample was stored in a 1.5 mL microtube. Please click here to view a larger version of this figure.
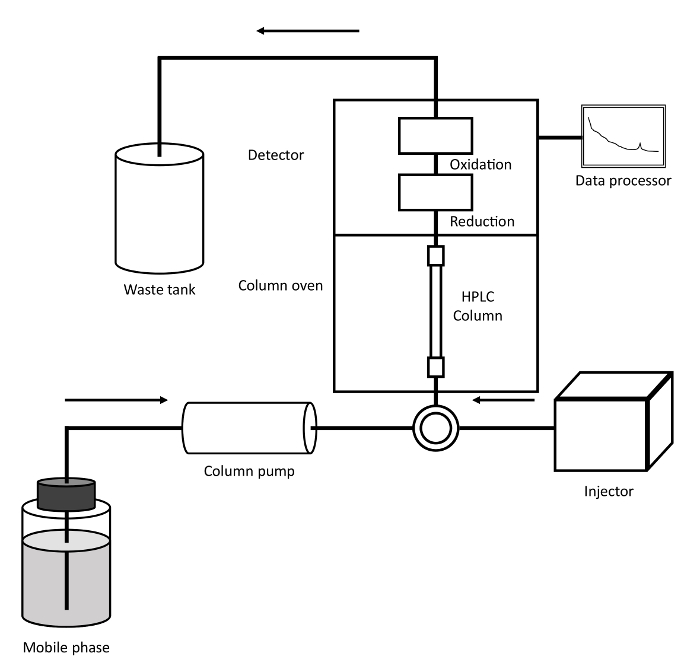
Figure 3: Schematic diagram of the HPLC-ECD system. 3-NT in an injected sample was separated in the HPLC column through which mobile phase flows. The 3-NT was reduced to aminotyrosine, oxidized to iminotyrosine, and detected electrochemically in the detector. Please click here to view a larger version of this figure.
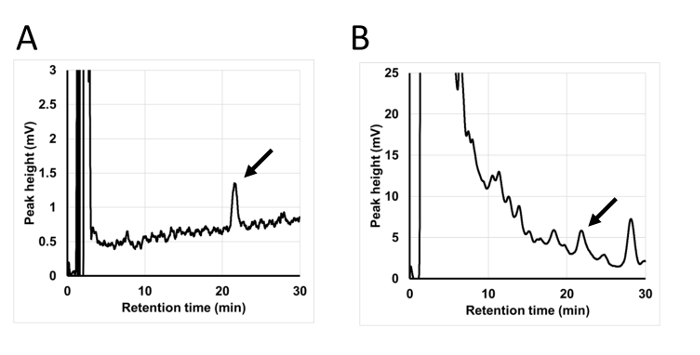
Figure 4: Typical chromatograms in the HPLC-ECD system. (A) Pure 3-NT analyzed by the HPLC-ECD system. (B) 6 mm circle sample from a PM7 filter used to detect 3-NT. A 3-NT peak is indicated by an arrow. Please click here to view a larger version of this figure.
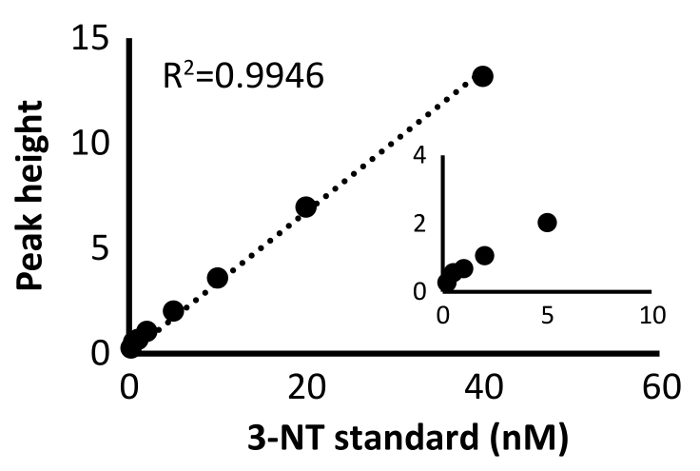
Figure 5: Standard curve of pure 3-NT samples. The results at a lower concentration are indicated in another graph. Please click here to view a larger version of this figure.
| Subject | Days of Collection | Spot | 3-NT (pg/m3 air) | |
| PM2.5 | Prefilter #1 | 27 | 4 | 8.25 ± 0.37 |
| Prefilter #2 | 27 | 4 | 29.66 ± 1.94 | |
| Prefilter #3 | 27 | 4 | 74.37 ± 4.09 | |
| Prefilter #4 | 27 | 4 | 32.70 ± 0.75 | |
| Backfilter | 7 | 5 | 4.21 ± 0.91 | |
| PM7 | Backfilter | 7 | 1 | 89.16 ± 6.36 |
| TSP | Filter | 7 | 1 | 10.34 ± 2.09 |
Table 1: 3-NT in the atmosphere measured by the HPLC-ECD system. The 6 mm circular spots from each filter were measured using the HPLC-ECD system. Prefilters #1 - #4 were collected in the same period. Note that the concentrations of 3-NT in the air are influenced by various factors, such as the collected date, year, or location. The data (n = 3 - 10) are represented as the mean ± the standard error.
Discussion
This article describes a quantification method to evaluate 3-NT in airborne PM collected on quartz filters using highly sensitive HPLC-ECD techniques.
In general, 3-NT measurement methods have been developed as biomarkers of oxidative stress in human diseases. Antibody-based methods (e.g., the enzyme-linked immunosorbent assay) are considered as semi-quantitative because there is no strict assay validation and it is difficult to assess the test's reliability. HPLC with electrochemical detection (ECD) and mass spectrometry-based assays have adequate sensitivity for the quantification of 3-NT13. Although they are sensitive, mass spectrometry-based methods like GC-MS or GC-MS/MS require the derivatization of amino acids, and the process of derivatization often results in the formation of artifacts14.
Compared to both the GC and LC techniques, HPLC-ECD is relatively less expensive and has a sufficient sensitivity to measure 3-NT. Additionally, the derivatization step in GC-MS and LC-MS often requires additional time. Although the method presented here requires 16 hours for the protein digestion step, it can, nevertheless, be carried out overnight (as described above, about 30 minutes of hands-on time is required per sample).Automatic repeat measurement by using an autosampler may provide a high-throughput measurement system.Previous studies have reported immunological methods to measure 3-NT in PM2,7; however, such methods could not detect 3-NT in the winter season due to the low levels of 3-NT in the PM15.
The HPLC-ECD method has additional advantages over other standard methods; for example, (i) an extraction process is not required in this method, (ii) it is completely detergent-free, (iii) it has minimal sample requirement, and, importantly, (iv) it has high sensitivity. Particles that are collected onto filters are frequently separated by sonication for further investigations, including the quantification of various components. Detergents are also used to isolate PM-bound proteins from filters. However, these additional steps increase the risk of contamination, sample loss, and underestimation due to extraction efficiency, and furthermore, most detergents are incompatible with LC/MS. In the present HPLC-ECD method, HPLC samples can easily be separated from the filter by using a simple hollow punch and without the requirement of an additional sample preparation process. The area of the round-shaped sample is 28.3 mm2, which is very small compared to the size of the original quartz filter (8 x 10 inch = 203.2 x 254 mm = 51,600 mm2).
The HPLC-ECD condition has been reviewed carefully to avoid interference to the 3-NT signal, as described previously12. A strong acidic pH of mobile phase and an adequate concentration of acetonitrile is important for detection.Using these conditions, other compounds, including nitro base, can be separated from nitrotyrosine.The present condition is suitable for the detection of 3-NT from samples with a different physical property (e.g., particulate matter and plasma).
To evaluate 3-NT in the air, the measurement of the filter weight and the elimination of the background are critical steps. In general, over approximately 100 mg of PM7 are collected over a period of four to seven days; however, several factors affect the filter weight before and after the PM7 collection, including humidity and static electrical charge. To avoid these effects, the stabilization of the filter weight for long periods is necessary under subequal temperature and humidity. The location where the electronic balance is installed should be maintained in a stable environment. For sample preparation, it is important to correct the background effects from the nonspecific protease cocktail and the ultrafiltration membrane, which often show a similar peak to 3-NT.
This method can be applied to other particles, including those in indoor environments. The nitration of proteins may increase their allergenic potential2. Therefore, this method may help to evaluate environmental cleanliness and prevent nitrosative stress.
In this study, we report a highly sensitive measurement method for atmospheric 3-NT of air sampler filters with easy-to-handle and inexpensive apparatus. The generation of 3-NT in the atmosphere is associated with environmental pollutants such as O3, NO2, PM proteins, and meteorological elements which affect human allergenicity. In conclusion, the method developed in the present investigation may help to assess the atmospheric reaction of O3, various pollutants, and PM proteins under various meteorological conditions. With these endeavors of developing HPLC-ECD for an estimation of 3-nitrotyrosine in the atmosphere, we anticipate better environmental cleanliness, improving human health.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Masayuki Kubo of the US Environmental Protection Agency for his help with the TSP/PM2.5 sampling. This work was supported in part by JSPS KAKENHI Grant Number JP16K15373 and JP18H03039.
Materials
| Name | Company | Catalog Number | Comments |
| Quartz filter, backup quartz filter | PALL life sciences | 7204 | Tissuquatrz 2500QAT-UP, 8x10in |
| High-volume air sampler | SIBATA SCIENTIFIC TECHNOLOGY | HV-1000F | |
| Particle size selector for SPM | SIBATA SCIENTIFIC TECHNOLOGY | 080130-061 | SPM (suspended particulate matters) is defined in Japan that particles with 10 um of 100% cut-off diameter. SPM is almost identical to PM7. |
| Low-volume air sampler | SIBATA SCIENTIFIC TECHNOLOGY | AH-600F | |
| Size classification unit for PM2.5 | SIBATA SCIENTIFIC TECHNOLOGY | AH-600 | |
| Quartz filters for classification (PM4.5–2.5, PM7.3–4.5, PM15–7.3, and TSP-PM15) | Tokyo Dylec | AHQ-630 | |
| Non-specific protease cocktail | Roche | 11459643001 | Pronase from Streptomyces griseus |
| 3-Nitrotyrosine | SIGMA | N7389 | |
| Ultrafiltration membranes | Millipore | UFC5010BK | AMICON ULTRA 0.5 mL - 10kDa cutoff |
| ECD | Eicom | HTEC-500, PEC-510 | standalone HPLC-ECD unit (HTEC-510) and Pre-electrolysis cell (PEC-510), includes pump |
| HPLC column | Eicom | SC-5ODS | |
| Data processor | Eicom | EPC-300 | |
| Injector | Hitachi High-Tech Science | L-7200 | Auto sampler |
| Analyzing software | eDAQ Japan | ES280 | PowerChrom software |
References
- Castillo, J. A., Staton, S. J. R., Taylor, T. J., Herckes, P., Hayes, M. A. Exploring the feasibility of bioaerosol analysis as a novel fingerprinting technique. Analytical and Bioanalytical Chemistry. 403 (1), 15-26 (2012).
- Franze, T., Weller, M. G., Niessner, R., Pöschl, U. Protein nitration by polluted air. Environmental Science & Technology. 39 (6), 1673-1678 (2005).
- Abe, R. Y., Akutsu, Y., Kagemoto, H. Protein amino acids as markers for biological sources in urban aerosols. Environmental Chemistry Letters. 14 (1), 155-161 (2016).
- D'Amato, G., et al. Allergenic pollen and pollen allergy in Europe. Allergy: European Journal of Allergy and Clinical Immunology. 62 (9), 976-990 (2007).
- Bolzacchini, E., et al. Gas-phase reaction of phenol with NO3. Environmental Science & Technology. 35 (9), 1791-1797 (2001).
- Shiraiwa, M., et al. Multiphase chemical kinetics of the nitration of aerosolized protein by ozone and nitrogen dioxide. Environmental Science & Technology. 46 (12), 6672-6680 (2012).
- Gruijthuijsen, Y. K., et al. Nitration enhances the allergenic potential of proteins. International Archives of Allergy and Immunology. 141 (3), 265-275 (2006).
- Kubo, M., Ogino, K., Tsukahara, H., Kaneko, K. Analytical Procedures for Nitrative/Nitrosative Stress. Studies on Pediatric Disorders. , 149-158 (2014).
- Thomson, L. 3-nitrotyrosine modified proteins in atherosclerosis. Disease Markers. 2015, 708282 (2015).
- Kuhn, D. M., Sakowski, S. A., Sadidi, M., Geddes, T. J. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons?. Journal of Neurochemistry. 89 (3), 529-536 (2004).
- Teixeira, D., Fernandes, R., Prudêncio, C., Vieira, M. 3-Nitrotyrosine quantification methods: Current concepts and future challenges. Biochimie. 125, 1-11 (2016).
- Hitomi, Y. H., et al. Disposition of protein-bound 3-nitrotyrosine in rat plasma analysed by a novel protocol for HPLC-ECD. Journal of Biochemistry. 141 (4), 495-502 (2007).
- Ogino, K., Wang, D. -. H. Biomarkers of oxidative/nitrosative stress: an approach to disease prevention. Acta Medica Okayama. 61 (4), 181-189 (2007).
- Tsikas, D., Mitschke, A., Suchy, M. -. T., Gutzki, F. -. M., Stichtenoth, D. O. Determination of 3-nitrotyrosine in human urine at the basal state by gas chromatography-tandem mass spectrometry and evaluation of the excretion after oral intake. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 827 (1), 146-156 (2005).
- Ito, T., Ogino, K., Nagaoka, K., Takemoto, K. Relationship of particulate matter and ozone with 3-nitrotyrosine in the atmosphere. Environmental Pollution. 236, 948-952 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved