A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Data Acquisition and Analysis In Brainstem Evoked Response Audiometry In Mice
In This Article
Summary
Brainstem evoked response audiometry is an important tool in clinical neurophysiology. Nowadays, brainstem evoked response audiometry is also applied in the basic science and preclinical studies involving both pharmacological and genetic animal models. Here we provide a detailed description of how auditory brainstem responses can be successfully recorded and analyzed in mice.
Abstract
Brainstem evoked response audiometry (BERA) is of central relevance in the clinical neurophysiology. As other evoked potential (EP) techniques, such as visually evoked potentials (VEPs) or somatosensory evoked potentials (SEPs), the auditory evoked potentials (AEPs) are triggered by the repetitive presentation of identical stimuli, the electroencephalographic (EEG) response of which is subsequently averaged resulting in distinct positive (p) and negative (n) deflections. In humans, both the amplitude and the latency of individual peaks can be used to characterize alterations in synchronization and conduction velocity in the underlying neuronal circuitries. Importantly, AEPs are also applied in basic and preclinical science to identify and characterize the auditory function in pharmacological and genetic animal models. Even more, animal models in combination with pharmacological testing are utilized to investigate for potential benefits in the treatment of sensorineural hearing loss (e.g., age- or noise-induced hearing deficits). Here we provide a detailed and integrative description of how to record auditory brainstem-evoked responses (ABRs) in mice using click and tone-burst application. A specific focus of this protocol is on pre-experimental animal housing, anesthesia, ABR recording, ABR filtering processes, automated wavelet-based amplitude growth function analysis, and latency detection.
Introduction
A central aspect of brain physiology is its capability to process environmental information resulting in different intrinsic or extrinsic output, such as learning, memory, emotional reactions, or motoric responses. Various experimental and diagnostic approaches can be used to characterize the electrophysiological responsiveness of individual neuronal cell types or clusters/ensembles of neurons within a stimulus-related neuronal circuitry. These electrophysiological techniques cover different spatiotemporal dimensions on the micro-, meso- and macroscale1. The microscale level includes voltage and current clamp approaches in different patch-clamp modes using, for instance, cultured or acutely dissociated neurons1. These in vitro techniques allow for the characterization of individual current entities and their pharmacological modulation2,3. An essential drawback, however, is the lack of systemic information as regards micro- and macrocircuitry information integration and processing. This impairment is partially overcome by in vitro techniques of the mesoscale, such as multielectrode arrays which allow for simultaneous extracellular multielectrode recordings not only in cultured neurons but also in acute brain slices4,5. Whereas microcircuitries can be preserved in the brain slices to a specific extent (e.g., in the hippocampus), long-range interconnections are typically lost6. Ultimately, to study the functional interconnections within neuronal circuitries, systemic in vivo electrophysiological techniques on the macroscale are the method of choice7. These approaches include, among other things, surface (epidural) and deep (intracerebral) EEG recordings which are carried out in both humans and animal models1. EEG signals are predominantly based on the synchronized synaptic input on pyramidal neurons in different cortical layers that can be inhibitory or excitatory in principal, despite the general predominance of excitatory input8. Upon synchronization, excitatory postsynaptic potential-based shifts in extracellular electrical fields are summed to form a signal of sufficient strength to be recorded on the scalp using surface electrodes. Notably, a detectable scalp recording from an individual electrode requires the activity of ten thousand of pyramidal neurons and a complex armamentarium of technical devices and processing tools, including an amplifier, filtering processes (low-pass filter, high-pass filter, notch filter), and electrodes with specific conductor properties.
In most experimental animal species (i.e., mice and rats), the human-based scalp EEG approach is technically not applicable, as the signal generated by the underlying cortex is too weak due to the limited number of synchronized pyramidal neurons9,10,11. In rodents, surface (scalp) electrodes or subdermal electrodes are thus severely contaminated by electrocardiogram and predominately electromyogram artifacts that make high-quality EEG recordings impossible9,11,12. When using unanesthetized freely moving mice and rats, it is therefore mandatory to directly record either from the cortex via epidural electrodes or from the deep, intracerebral structures to ensure the direct physical connection of the sensing tip of the lead/implanted electrode to the signal-generating neuronal cell clusters. These EEG approaches can be carried out either in a restraining tethered system setup or using the nonrestraining implantable EEG radio telemetry approach9,10,11. Both techniques have their pros and cons and can be a valuable approach in the qualitative and quantitative characterization of seizure susceptibility/seizure activity, circadian rhythmicity, sleep architecture, oscillatory activity, and synchronization, including time-frequency analysis, source analysis, etc.9,10,13,14,15,16,17.
Whereas tethered systems and radio telemetry allow for EEG recordings under restraining/semirestraining or nonrestraining conditions, respectively, related experimental conditions do not match the requirements for ABR recordings. The latter demand for defined acoustic stimuli which are presented repetitively over time with defined positions of a loudspeaker and experimental animal and controlled sound pressure levels (SPLs). This can be achieved either by head fixation under restraining conditions or following anesthesia18,19. To reduce the experimental stress, animals are normally anesthetized during ABR experimentation, but it should be considered that anesthesia can interfere with ABRs19,20.
As a general characteristic, the EEG is built up of different frequencies in a voltage range of 50-100 µV. Background frequencies and amplitudes strongly depend on the physiological state of the experimental animal. In the awake state, beta (β) and gamma (γ) frequencies with lower amplitude predominate. When animals become drowsy or fall asleep, alpha (α), theta (θ), and delta (δ) frequencies arise, exhibiting increased EEG amplitude21. Once a sensory channel (e.g., the acoustic pathway) is stimulated, information propagation is mediated via neuronal activity through the peripheral and central nervous system. Such sensory (e.g., acoustic) stimulation triggers so-called EPs or evoked responses. Notably, event-related potentials (ERPs) are much lower in amplitude than the EEG (i.e., a few microvolts only). Thus, any individual ERP based on a single stimulus would be lost against the higher-amplitude EEG background. Therefore, a recording of an ERP requires the repetitive application of identical stimuli (e.g., clicks in ABR recordings) and subsequent averaging to eliminate any EEG background activity and artifacts. If ABR recordings are done in anesthetized animals, it is easy to use subdermal electrodes here.
Principally, AEPs include short-latency EPs, which are normally related to ABRs or BERA, and further, later-onset potentials such as midlatency EPs (midlatency responses [MLR]) and long-latency EPs22. Importantly, disturbance in the information processing of the auditory information is often a central feature of neuropsychiatric diseases (demyelinating diseases, schizophrenia, etc.) and associated with AEP alterations23,24,25. Whereas behavioral investigations are only capable of revealing functional impairment, AEP studies allow for precise spatiotemporal analysis of auditory dysfunction related to specific neuroanatomical structures26.
ABRs as early, short-latency acoustically EPs are normally detected upon moderate to high-intense click application, and there may occur up to seven ABR peaks (WI-WVII). The most important waves (WI-WV) are related to the following neuroanatomical structures: WI to the auditory nerve (distal portion, within the inner ear); WII to the cochlear nucleus (proximal portion of the auditory nerve, brainstem termination); WIII to the superior olivary complex (SOC); WIV to the lateral lemniscus (LL); WV to the termination of the lateral lemniscus (LL) within the inferior colliculus (IC) on the contralateral side27 (Supplementary Figure 1). It should be noted that WII-WV are likely to have more than one anatomical structure of the ascending auditory pathway contributing to them. Notably, the exact correlation of peaks and underlying structures of the auditory tract is still not fully clarified.
In audiology, ABRs can be used as a screening and diagnostic tool and for surgical monitoring28,29. It is most important for the identification of dysacusis, hypacusis, and anacusis (e.g., in age-related hearing loss, noise-induced hearing loss, metabolic and congenital hearing loss, and asymmetric hearing loss and hearing deficits due to deformities or malformations, injuries, and neoplasms)28. ABRs are also relevant as a screening test for hyperactive, intellectually impaired children or for other children who would not be able to respond to conventional audiometry (e.g., in neurological/psychiatric diseases such as ADHD, MS, autism etc.29,30) and in the development and surgical fitting of cochlear implants28. Finally, ABRs can provide valuable insight into the potential ototoxic side-effects of neuropsychopharmaceuticals, such as antiepileptics31,32.
The value of the translation of neurophysiological knowledge obtained from pharmacological or transgenic mouse models to humans has been demonstrated in numerous settings, particularly on the level of ERPs in auditory paradigms in mice and rats33,34,35. New insight into altered early AEPs and associated changes in auditory information processing in mice and rats can thus be translated to humans and is of central importance in the characterization and endophenotyping of auditory, neurological, and neuropsychiatric diseases in the future. Here we provide a detailed description of how ABRs can be successfully recorded and analyzed in mice for basic scientific, toxicological, and pharmacological purposes.
Protocol
All animal procedures were performed according to the guidelines of the German Council on Animal Care and all protocols were approved by the local institutional and national committee on animal care (Landesamt für Natur, Umwelt, und Verbraucherschutz, State Office of North Rhine-Westphalia, Department of Nature, Environment and Consumerism [LANUV NRW], Germany). The authors further certify that all animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 or the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, or the European Communities Council Directive of November 24, 1986 (86/609/EEC) and of September 22, 2010 (2010/63/EU). Specific effort was made to minimize the number of animals used and their suffering (3R [replacement, reduction, and refinement] strategy).
1. Experimental animals
- Selection of experimental animals and species
- Perform ABR studies in rodents/rodent models (i.e. mice or rats) that fulfill the requirements of homology, isomorphism, and predictability related to a specific human disease. This is of specific importance in terms of basic aspects in translational neuroscience.
NOTE: Consider that available miscellaneous mouse and rat strains can show differences in basic physiological and pathophysiological characteristics36,37,38. These mouse/rat line-related specificities have to be taken into account in experimental planning. - Consider mouse- and rat-strain-specific alterations in physiology and pharmacology that might have an impact on electrophysiological experiments (e.g. altered anesthetic sensitivities, circadian rhythmicity, [audiogenic] seizure susceptibility, age, and genetic background)39,40,41,42.
- Include the gender-specific stratification in the study design. Remember that the estrous cycle can severely affect anesthetic susceptibility, central rhythmicity, circadian dependency, and seizure activity (auditory seizures) and sensory (auditory) information processing43,44,45. Thus, perform a gender-specific analysis.
NOTE: Restrict to male mice if the financial and experimental capacity is limited, although various neurophysiological parameters from normally cycling females do not seem to exhibit increased variability compared to males46.
- Perform ABR studies in rodents/rodent models (i.e. mice or rats) that fulfill the requirements of homology, isomorphism, and predictability related to a specific human disease. This is of specific importance in terms of basic aspects in translational neuroscience.
- Animal housing and handling
- House mice or rats in individually ventilated cages inside an animal facility.
- Move the experimental animals from the animal facility to ventilated cabinets located in special lab rooms intended for anesthesia, ABR electrode placement, and ABR recordings.
- Make sure that the animals are housed in a ventilated cabinet under standard environmental conditions (i.e., with a temperature of 21 ± 2 °C, 50%-60% relative humidity, and a conventional 12/12 h light/dark cycle). Allow the animals to acclimatize and adapt to this circadian pattern for at least 14 days prior to subsequent experimentation.
- Use clear polycarbonate cages type II (26.7 cm x 20.7 cm x 14.0 cm, an area of 410 cm2) for housing mice in groups of 3-4 and use clear polycarbonate cages type III (42.5 cm x 26.6 cm x 18.5 cm, an area of 800 cm2) for rats. Provide ad libitum access to drinking water and standard food pellets.
- Avoid separation/isolation of the experimental animals prior to and after ABR recordings as isolation can exert severe stress affecting experimental results. Thus, place the animals back into their home cage following anesthesia, ABR electrode placement, and ABR recordings.
- Do not apply open housing conditions as they are subject to a variety of experimental drawbacks, particularly in auditory studies. Ventilated cabinets, instead, protect from acoustic stress prior to and in between experimental auditory procedures which could otherwise lead to sensorineural hearing loss (e.g., noise-induced hearing loss) and thus affect results.
- Utilize mouse- and rat-specific sanitary, anesthetic, and technical equipment so that neither mice nor rats can sense the presence of each other as mutual sensory perception of rivaling species may give rise to avoidable confounding factors in the studies.
2. Mouse anesthesia
- Perform anesthesia using injectable anesthetics. Prepare a combination of ketamine hydrochloride (rodent dosage: 100 mg/kg) and xylazine hydrochloride (rodent dosage: 10 mg/kg) in 0.9% NaCl or Ringer solution and inject the animal intraperitoneally based on its body weight.
NOTE: Inhalation narcosis via isoflurane is not recommended as the ABR procedure normally requires a sound attenuating cubicle and Faraday cage, resulting in spatial limitations within the recording setup. Although many anesthetics act on the NMDA system and obviously influence ABR recording outcomes, a non-anesthetic restraining approach in ABR recordings is not recommended as restraining procedures under consciousness induce dramatic stress to the animal, with severe subsequent artifact formation in ABRs. - Observe the animals carefully for the depth of anesthesia by performing a tail pinch, foot pinch, and monitoring respiration rate (mice: 150-220 breaths/min). Check for possible gasping and counteract if necessary.
NOTE: Different mouse lines or pharmacological mouse models can exhibit different sensitivities to anesthesia. The same holds true for mutant mouse models. Endotracheal intubation is not a must in this experimental setting and not recommended. As intubation increases the risk of trauma to the trachea and infection, the benefit/risk of endotracheal intubation during the ABR procedure is negative.
3. General aspects of perianesthetic arrangements and instrumentation
- Apply supplemental warmth during and after ABR recordings using a homeothermic heating blanket to maintain the animal’s body core temperature. Maintain the latter at 36.5-38.0 °C (98.6-100.4 °F).
NOTE: Hypothermia is a risk factor in small rodents due to their high ratio of body surface (mouse body surface = 10.5 x (weight in g)2/3; rat body surface = 10.5 x (weight in g)2/3) to the body volume. - Cover the animal’s eyes with petroleum-based artificial tear ointment or 5% dexpanthenol during the entire ABR recording process to avoid corneal desiccation. Continue this procedure until the blinking reflex is fully restored.
- Sterilize the experimental instruments (see the Table of Materials) using an autoclave or disinfectants.
NOTE: The usage of a heat-based surgical instrument sterilizer with glass beads is recommended. - For exact ABR electrode placement, use a binocular surgical magnification microscope with a cold light source for the intense illumination via flexible or self-supporting movable light guides.
- Use a clean laboratory coat, a facemask, a head cover, and sterile gloves during experimental animal handling and experimentation.
NOTE: Optimal instruments and supplies can vary between labs and must meet lab-specific and institutional standards.
4. ABR recordings
NOTE: The protocol described here is based on a commercially available ABR system for monaural and binaural recordings. Importantly, the scientific question to be addressed must meet the technical specifications of the ABR system used. ABR analysis of binaural recordings, for example, can be used to investigate the lateral coding of auditory stimuli in the auditory pathway and to study peripheral lateral asymmetry in neuropsychiatric diseases.
- Perform a calibration of stimulation frequencies on each day of recording by placing a microphone connected to a preamplifier and the processing system (see the Table of Materials) inside the sound attenuating cubicle at the location with the correct orientation where the experimental murine ear will be positioned.
- Turn on the preamplifier connected to the microphone at least 5 min prior to the calibration to allow for the equilibration of the system.
- Turn on the oscilloscope.
- Position the microphone connected to a preamplifier inside the sound attenuating cubicle to mimic the experimental murine ear.
- Open the commercially available processing and acquisition software (see the Table of Materials).
- Select the calibration Cal200K file within the software to activate the calibration-configuration mode and choose parameters according to the experimental conditions.
- Use the processor system to execute the calibration procedure. Make sure that the technical specifications of the microphone and loudspeaker in terms of SPL limits, frequency range, and distribution harmonize.
- Select and start the predefined click stimulation protocol.
- Run a single click SPL (preferably, the maximum SPL) to verify that the spectrum of sound stimuli as analyzed by online Fast Fourier Transformation (FFT) of the oscilloscope matches requirements (substantial energy range).
- Select and start the predefined tone-burst stimulation protocol within range of interest (e.g., 1-42 kHz).
- Confirm the frequency spectrum of the recorded acoustic test stimuli by using an oscilloscope and online FFT.
NOTE: Daily calibration of the system and stimulation frequencies is necessary to guarantee that the stimulation frequencies and SPLs are within acceptable working ranges.
- Place the anesthetized mouse inside a sound attenuating cubicle lined with acoustical foam.
NOTE: The entire cubicle should be covered by a Faraday cage (custom-made meshed metal or a commercial one) to shield the ABR recordings from external electrical interference and protect them from noise. - For the recording of monaural brainstem-evoked auditory potentials, insert subdermal stainless-steel electrodes at the vertex, axial of the pinnae (positive [+] electrode) and ventrolateral of the right or left pinna (negative [-] electrode) depending on the ear to be measured. For binaural recordings, place the negative electrodes at both the right and left pinnae. Position the ground electrode at the hip of the animal (Supplementary Figure 1).
- Prior to the insertion, form a hook shape at the tip of the stainless-steel electrode so that subdermal fixation of the electrodes is guaranteed47.
- Perform impedance measurements of all electrodes prior to each recording to verify proper electrode positioning/conductivity. Use the impedance check button on the four-channel headstage to verify each electrode impedance level.
NOTE: Impedance should be less than 5 kΩ. - Record ABRs under free-field conditions using a single loudspeaker (frequency bandwidth, for instance, at 1-65 kHz) placed 10 cm opposite to the rostrum of the animals (the loudspeaker’s leading edge perpendicular to the mouse’s interaural axis). Make sure that the position of the mouse head/mouse ears is that of the calibration microphone, depending on the chosen specific distance between the loudspeaker and the microphone during calibration.
NOTE: Instead of free-field conditions, ear tubing can also be used. However, special precautions and tests are necessary to determine SPLs in these settings. - Program the stimulus protocols for the clicks and tone bursts using self-programmed or commercially available software (see the Table of Materials). The individual stimulus parameters listed below need to be added to the related graphical user interface.
- Start with the configuration of the click stimulus entity (i.e., a 100 μs duration stimulus with alternating polarity [switching between condensation and rarefaction] and the defined substantial energy. Use this stimulus entity to analyze and determine click thresholds, ABR symmetry of the left and right ear, ABR W (I - IV) amplitudes, and W (I - IV) latencies later on.
- Initiate the software and use the configuration window to add the click stimulus parameters. Click Execute to run the protocol.
- Continue with the configuration of the second stimulus entity, which is a 4.5 ms tone burst (transient sinusoidal pulse) of alternating polarity with Hann envelope rise and fall times of 1.5 ms each (gate/ramp time duration). Consider a minimum tone burst duration of 3 ms, particularly for low-frequency tone bursts. Use this stimulus to analyze and identify frequency-specific hearing thresholds in all genotypes.
- Similar to step 4.6.2, use the configuration window to add tone burst stimulus parameters and click Execute to run the protocol (as stated by the manufacturer48).
- For tone burst studies, program the appropriate frequency range to be tested depending on the scientific question (e.g., from 1-42 kHz in 6 kHz steps). Make sure that the frequency ranges to be applied meet the technical capabilities of the loudspeaker (in this case, a multifield magnetic speaker with a frequency bandwidth of 1-65 kHz for free- or closed-field conditions).
- For averaging, set the number of sequential acoustic stimuli (clicks or tone bursts), for instance, at 300x with a rate of 20 Hz.
- Increase the SPLs in 5 dB steps for clicks and 10 dB steps for tone bursts, starting from 0 dB up to 90 dB (increasing SPL mode).
NOTE: Both increasing and decreasing SPL modes have been described in the literature. SPL step size might be adapted due to scientific questions.
- Determine an ABR data acquisition duration of 25 ms, starting with a 5 ms baseline period prior to the individual acoustic stimulus onset (pre-ABR baseline) and exceeding a 10 ms ABR section by another 10 ms baseline (post-ABR baseline) (Supplementary Figure 1).
- Apply an appropriate sampling rate for ABR data acquisition (e.g., 24.4 kHz) and bandpass filter (high pass: 300 Hz, low pass: 5 kHz) using a 6-pole Butterworth filter. Activate the notch filter if necessary.
NOTE: Sampling rate and filter characteristics might be adapted due to experimental requirements. - Transfer the resultant bioelectrical signals recorded from the subdermal electrodes to a head stage and further forward to a preamplifier with appropriate amplification (e.g., 20-fold).
- Use a specific ABR system processing software to coordinate loudspeaker control and ABR acquisition, processing, averaging, and data management.
- Try to execute the entire ABR protocols (for click- and tone burst-evoked hearing thresholds, peak amplitude, and peak latency analysis, etc.) within about 45 min. This corresponds to the time of deep narcosis using 100/10 mg ketamine/xylazine intraperitoneally.
- Make sure that the calibration, programming/adjustments for stimulus presentation and acquisition, filter settings, etc. are working as expected prior to anesthetizing the animal and performing the actual recording.
5. ABR analysis
- Click- and tone burst-evoked ABR hearing threshold analysis
- Perform automated threshold detection based on earlier publications to avoid potential inconsistencies in the ABR threshold determination by visual inspection/estimation49,50,51,52.
- Define three distinct time windows (TWs) to calculate the signal-to-noise ratio (SNR): TW1 (0-5 ms), TW2 (5-15 ms), and TW3 (15-25 ms) (Supplementary Figure 1).
- Calculate the noise standard deviation of the baseline within the two distinct TWs (i.e., TW1 and TW3) where no AEPs are observed. This calculation can be done using self-programmed software.
- Calculate for each SPL measurement within an ABR record setting both the mean and the standard deviation for the pooled data of TW1 and TW3.
- Reset all recording samples individually by the corresponding calculated mean to remove any DC offset.
- For hearing threshold determination, identify the lowest SPL (dB) where at least one wave amplitude value (WI-WIV) in the ABR response time window (TW2) exceeded the fourfold of the previously calculated standard deviation.
NOTE: If no ABR wave was detected for click and frequency threshold analysis at the maximum SPL, a nominal threshold level of 100dB is assigned to the ear.
- ABR wave amplitude and wave latency analysis
- Conduct a wavelet-based approach using the Mexican hat wavelet to determine the temporal-sequential arrangement of positive (p) waves (peaks) as well as the negative (n) waves (pits) using a default wavelet by the continuous wavelet transform (CWT)-based pattern-matching algorithm52 (Supplementary Figure 1).
- Mathematically, the CWT is represented as follows53.
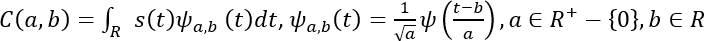
Here, s(t) is the signal, a is the scale, b is the translation, ψ(t) is the mother wavelet, ψa, b(t) is the scaled and translated wavelet, and C is the 2D matrix of wavelet coefficients.
- Mathematically, the CWT is represented as follows53.
- Initially, use the 55-dB measurement of each ABR run to identify the best scale parameters for each wave to be passed to the CWT, which results in three classes: scales 0.5-4 for all n-waves, 0.5-6 for all p-waves, and 0.5-12 for WIV as this is the broadest wave within the samples.
NOTE: The 55 dB SPL was chosen as waves are generally most prominent here and can be reliably detected. - Prove all classes to reliably detect the correct temporal collocation of WI-WIV within all 55 dB measurements.
- To determine ABR WI-WIV in the accurate temporal order within the 55 dB measurement, p-peaks and n-peaks (pits) are identified in a fixed sequence using relative positions of previously identified peaks to limit the time window of subsequent scans.
- Once all nine peaks are identified at 55 dB, use related values as starting points for the temporal search frame for the adjacent sound pressure measurements (50 dB and 60 dB) before the identification of peaks 1-9 is repeated.
- In this manner, determine p- and n-peaks of all dB levels (55-0 dB and 60-90 dB) if possible. Once a p- and n-peak is no longer identified by the wavelet analysis, its temporal arrangement is set by calculating the temporal offset of the peak to any other peak identified in the previous dB level.
- Applying the temporal offset to peaks to any other p- and n-peak within the current decibel level results in a maximum of eight determined temporal positions for the undefined peaks whereof the mean is taken as the closest approximation.
- To evaluate the amplitude growth function and latency comparison of all waves (WI-WIV), characterize the maximum amplitudes and mean latencies of each of the p-peaks within the time frame of the related n-peaks.
- Visually check all results based on the self-programmed automatic wavelet tool afterward, and, if necessary, exclude individual ABR runs from the statistics if they do not meet the strict inclusion/quality criteria.
NOTE: In both automated analysis and visual inspection of ABRs, a double-blinded approach is recommended.
- Conduct a wavelet-based approach using the Mexican hat wavelet to determine the temporal-sequential arrangement of positive (p) waves (peaks) as well as the negative (n) waves (pits) using a default wavelet by the continuous wavelet transform (CWT)-based pattern-matching algorithm52 (Supplementary Figure 1).
6. Post-operative care and post-ABR treatment
- Continuously monitor the animals until they have regained consciousness and are able to maintain sternal recumbency.
- Do not return an animal that has undergone ABR recordings to the company of other animals until it has fully recovered.
- Inject carprofen (mouse: 1x 5-10 mg/kg, subcutaneously; rat: 1x 2.5-5.0 mg/kg, subcutaneously) for post-operative pain treatment.
NOTE: Long-lasting pain treatment is not required as ABR recording electrodes are inserted subcutaneously. - Postoperatively, feed moistened pellets in order to facilitate food uptake. Carefully observe food (~15 g/100 g of body weight/day; ~5 g/24 h) and water (~15 mL/100 g of body weight/day; ~5 mL/24 h) consumption.
- Monitor the animals closely for the return of their normal postures and behavior.
NOTE: Systemic administration of antibiotics such as enrofloxacin or trimethoprim-sulfonamide is not recommended here, as subdermal electrode placement is of only minimal invasiveness. Application of antibiotics should be restricted unless signs of local or generalized inflammation occur. - Follow-up postexperimental recovery after ABR recordings by controlling the animal’s body weight.
Results
Click- and tone burst-evoked ABR recordings can be used to evaluate hearing threshold differences, amplitude growth function, and latency comparison. Click-evoked ABRs in the SPL increasing mode are depicted in Figure 1 for controls and two exemplary mutant mouse lines which are deficient for the Cav3.2 T-type voltage-gated Ca2+ channel (i.e., Cav3.2+/- and Cav3.2 null mutants [Cav3.2-/-
Discussion
This protocol provides a detailed and integrative description of how to record auditory evoked brainstem responses in mice. It puts specific focus on animal pretreatment, anesthesia, and potential methodological confounding factors. The latter include, among others, gender, mouse line, age, and housing conditions. It should be noted that all these factors can have an impact on sensorineural hearing loss and fundamental aspects of auditory information processing. Thus, appropriate stratification of auditory profiling stud...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Dr. Christina Kolb (German Center for Neurodegenerative Diseases [DZNE]) and Dr. Robert Stark (DZNE) for their assistance in the animal breeding and animal health care. This work was financially supported by the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM, Bonn, Germany).
Materials
| Name | Company | Catalog Number | Comments |
| AEP/OAE Software for RZ6 (BioSigRZ software) | Tucker-Davis Technologies (TDT) | BioSigRZ | |
| Binocular surgical magnification microscope | Zeiss Stemi 2000 | 0000001003877, 4355400000000, 0000001063306, 4170530000000, 4170959255000, 4551820000000, 4170959040000, 4170959050000 | |
| Cages (Macrolon) | Techniplast | 1264C, 1290D | |
| Carprox vet, 50mg/ml | Virbac Tierarzneimittel GmbH | PZN 11149509 | |
| Cold light source | Schott KL2500 LCD | 9.705 202 | |
| Cotton tip applicators (sterile) | Carl Roth | EH12.1 | |
| Custom made meshed metal Faraday cage (stainless steel, 2 mm thickness, 1 cm mesh size) | custom made | custom made | |
| 5% Dexpanthenole (Bepanthen eye and nose creme) | Bayer Vital GmbH | PZN: 01578681 | |
| Disposable Subdermal stainless steel Needle electrodes, 27GA, 12mm | Rochester Electro-Medical, Inc. | S03366-18 | |
| Surgical drape sheets (sterile) | Hartmann | PZN 0366787 | |
| Ethanol, 70% | Carl Roth | 9065.5 | |
| 1/4'' Free Field Measure Calibration Mic Kit | Tucker-Davis Technologies (TDT) | PCB-378C0 | |
| Gloves (sterile) | Unigloves | 1570 | |
| Graefe Forceps-curved, serrated | FST | 11052-10 | |
| GraphPad Prism 6 Software, V6.07 | GraphPad Prism Software, Inc. | https://www.graphpad.com/ | |
| Heat-based surgical instrument sterilizer | FST | 18000-50 | |
| Homeothermic heating blanked | ThermoLux | 461265 / -67 | |
| Ketanest S (Ketamine), 25mg/ml | Pfizer | PZN 08707288 | |
| Ringer’s solution (sterile) | B.Braun | PZN 01471434 | |
| Matlab software | MathWorks, Inc. | https://de.mathworks.com/products/matlab.html | |
| Medusa 4-Channel Low Imped. Headstage | Tucker-Davis Technologies (TDT) | RA4LI | |
| Medusa 4-Channel Pre-Amp/Digitizer | Tucker-Davis Technologies (TDT) | RA4PA | |
| Microphone | PCB Pieztronics | 378C01 | |
| Multi Field Speaker- Stereo | Tucker-Davis Technologies (TDT) | MF1-S | |
| Oscilloscope | Tektronix | DPO3012 | |
| Optical PC1 express card for Optibit Interface) | Tucker-Davis Systems (TDT) | PO5e | |
| Askina Braucel pads (cellulose absorbet pads) | B.Braun | PZN 8473637 | |
| Preamplifier | PCB Pieztronics | 480C02 | |
| RZ6 Multi I/O Processor system (BioSigRZ) | Tucker-Davis Technologies (TDT) | RZ6-A-PI | |
| 0.9% saline (NaCl, sterile) | B.Braun | PZN:8609255 | |
| SigGenRZ software | Tucker-Davis Technologies (TDT) | https://www.tdt.com/ | |
| Software R (version 3.2.1) + Reshape 2 (Version 1.4.1) + ggplot 2 (version 1.0.1) + datatable (version 1.9.4), + gdata (version 2.13.3), + pastecs (version 1.3.18), + waveslim (version 1.7.5), + MassSpecWavelet (version 1.30.0) | The R Foundation, R Core Team 2015 | Open Source Software (freely distributable) | |
| Sound attenuating cubicle | Med Associates Inc. | ENV-018V | |
| Standard Pattern Forceps, 12cm and 14.5 cm length | FST | 11000-12, 11000-14 | |
| Leukosilk tape | BSN medical GmbH & Co. KG | PZN 00397109 | |
| Tissue Forceps- 1x2 Teeth 12 cm | FST | 11021-12 | |
| Uniprotect ventilated cabinet | Bioscape | THF3378 | |
| Ventilated cabinet | Tecniplast | 9AV125P | |
| Xylazine (Rompun), 2% | Bayer Vital GmbH | PZN 1320422 |
References
- Sporns, O., Tononi, G., Kotter, R. The human connectome: A structural description of the human brain. PLOS Computational Biology. 1 (4), e42 (2005).
- Bebarova, M. Advances in patch clamp technique: towards higher quality and quantity. General Physiology and Biophysics. 31 (2), 131-140 (2012).
- Kornreich, B. G. The patch clamp technique: principles and technical considerations. Journal of Veterinary Cardiology. 9 (1), 25-37 (2007).
- Spira, M. E., Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nature Nanotechnology. 8 (2), 83-94 (2013).
- Obien, M. E., Deligkaris, K., Bullmann, T., Bakkum, D. J., Frey, U. Revealing neuronal function through microelectrode array recordings. Frontiers in Neuroscience. 8, 423 (2014).
- Heuschkel, M. O., Fejtl, M., Raggenbass, M., Bertrand, D., Renaud, P. A three-dimensional multi-electrode array for multi-site stimulation and recording in acute brain slices. Journal of Neuroscience Methods. 114 (2), 135-148 (2002).
- Kimiskidis, V. K. Transcranial magnetic stimulation (TMS) coupled with electroencephalography (EEG): Biomarker of the future. Reviews in Neurology. 172 (2), 123-126 (2016).
- Nunez, P. L. Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behavioral Brain Science. 23 (3), 371-437 (2000).
- Lundt, A., et al. EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research. Neural Plasticity. 2016, 8213878 (2016).
- Papazoglou, A., et al. Non-restraining EEG Radiotelemetry: Epidural and Deep Intracerebral Stereotaxic EEG Electrode Placement. Journal of Visualized Experiments. 112 (112), e54216 (2016).
- Weiergraber, M., Henry, M., Hescheler, J., Smyth, N., Schneider, T. Electrocorticographic and deep intracerebral EEG recording in mice using a telemetry system. Brain Research Brain Research Protocols. 14 (3), 154-164 (2005).
- Kallstrand, J., Nehlstedt, S. F., Skold, M. L., Nielzen, S. Lateral asymmetry and reduced forward masking effect in early brainstem auditory evoked responses in schizophrenia. Psychiatry Research. 196 (2-3), 188-193 (2012).
- Muller, R., et al. Automatic Detection of Highly Organized Theta Oscillations in the Murine EEG. Journal of Visualized Experiments. (121), e55089 (2017).
- Papazoglou, A., et al. Gender specific hippocampal whole genome transcriptome data from mice lacking the Cav2.3 R-type or Cav3.2 T-type voltage-gated calcium channel. Data in Brief. 12, 81-86 (2017).
- Papazoglou, A., et al. Gender-Specific Hippocampal Dysrhythmia and Aberrant Hippocampal and Cortical Excitability in the APPswePS1dE9 Model of Alzheimer's Disease. Neural Plasticity. 2016, 7167358 (2016).
- Papazoglou, A., et al. Motor Cortex Theta and Gamma Architecture in Young Adult APPswePS1dE9 Alzheimer Mice. PLOS ONE. 12 (1), e0169654 (2017).
- Siwek, M. E., et al. Altered theta oscillations and aberrant cortical excitatory activity in the 5XFAD model of Alzheimer's disease. Neural Plasticity. , 781731 (2015).
- Welch, T. M., Church, M. W., Shucard, D. W. A method for chronically recording brain-stem and cortical auditory evoked potentials from unanesthetized mice. Electroencephalography and Clinical Neurophysiology. 60 (1), 78-83 (1985).
- Church, M. W., Gritzke, R. Effects of ketamine anesthesia on the rat brain-stem auditory evoked potential as a function of dose and stimulus intensity. Electroencephalography and Clinical Neurophysiology. 67 (6), 570-583 (1987).
- van Looij, M. A., et al. Impact of conventional anesthesia on auditory brainstem responses in mice. Hearing Research. 193 (1-2), 75-82 (2004).
- Schomer, D. L., da Silva, F. L. . Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. , (2011).
- De Cosmo, G., Aceto, P., Clemente, A., Congedo, E. Auditory evoked potentials. Minerva Anestesiology. 70 (5), 293-297 (2004).
- Rosburg, T. Auditory N100 gating in patients with schizophrenia: A systematic meta-analysis. Clinical Neurophysiology. 129 (10), 2099-2111 (2018).
- DiLalla, L. F., McCrary, M., Diaz, E. A review of endophenotypes in schizophrenia and autism: The next phase for understanding genetic etiologies. American Journal of Medical Genetics Part C Seminar in Medical Genetics. 175 (3), 354-361 (2017).
- Walsh, P., Kane, N., Butler, S. The clinical role of evoked potentials. Journal of Neurology, Neurosurgery and Psychiatry. 76 Suppl 2, ii16-ii22 (2005).
- Opgen-Rhein, C., Neuhaus, A., Urbanek, C., Dettling, M. New strategies in schizophrenia: impact of endophentotypes. Psychiatrische Praxis. 31 Suppl 2, S194-S199 (2004).
- Knipper, M., Van Dijk, P., Nunes, I., Ruttiger, L., Zimmermann, U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Progress in Neurobiology. 111, 17-33 (2013).
- Miller, C. A., Brown, C. J., Abbas, P. J., Chi, S. L. The clinical application of potentials evoked from the peripheral auditory system. Hearing Research. 242 (1-2), 184-197 (2008).
- Manouilenko, I., Humble, M. B., Georgieva, J., Bejerot, S. Brainstem Auditory Evoked Potentials for diagnosing Autism Spectrum Disorder, ADHD and Schizophrenia Spectrum Disorders in adults. A blinded study. Psychiatry Research. 257, 21-26 (2017).
- Talge, N. M., Tudor, B. M., Kileny, P. R. Click-evoked auditory brainstem responses and autism spectrum disorder: A meta-analytic review. Autism Research. 11 (6), 916-927 (2018).
- Hamed, S. A. The auditory and vestibular toxicities induced by antiepileptic drugs. Expert Opinion in Drug Safety. 16 (11), 1281-1294 (2017).
- Ismi, O., et al. The Effect of Methylphenidate on the Hearing of Children with Attention Deficit Hyperactivity Disorder. International Archive in Otorhinolaryngology. 22 (3), 220-224 (2018).
- Michna, M., et al. Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice. Journal of Physiology. 553 (Pt 3), 747-758 (2003).
- Platzer, J., et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 102 (1), 89-97 (2000).
- Willaredt, M. A., Ebbers, L., Nothwang, H. G. Central auditory function of deafness genes. Hearing Research. 312, 9-20 (2014).
- Yee, B. K., Singer, P. A conceptual and practical guide to the behavioural evaluation of animal models of the symptomatology and therapy of schizophrenia. Cell Tissue Research. 354 (1), 221-246 (2013).
- Fahey, J. R., Katoh, H., Malcolm, R., Perez, A. V. The case for genetic monitoring of mice and rats used in biomedical research. Mammalian Genome. 24 (3-4), 89-94 (2013).
- Hunsaker, M. R. Comprehensive neurocognitive endophenotyping strategies for mouse models of genetic disorders. Progress in Neurobiology. 96 (2), 220-241 (2012).
- Turner, J. G., Parrish, J. L., Hughes, L. F., Toth, L. A., Caspary, D. M. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Computational Medicine. 55 (1), 12-23 (2005).
- Neumann, P. E., Collins, R. L. Genetic dissection of susceptibility to audiogenic seizures in inbred mice. Proceedings of the National Academy of Sciences of the United States of America. 88 (12), 5408-5412 (1991).
- Meier, S., Groeben, H., Mitzner, W., Brown, R. H. Genetic variability of induction and emergence times for inhalational anaesthetics. European Journal of Anaesthesiology. 25 (2), 113-117 (2008).
- Majewski-Tiedeken, C. R., Rabin, C. R., Siegel, S. J. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Dependence. 92 (1-3), 217-227 (2008).
- Bonthuis, P. J., et al. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Frontiers in Neuroendocrinology. 31 (3), 341-358 (2010).
- Buckmaster, P. S., Haney, M. M. Factors affecting outcomes of pilocarpine treatment in a mouse model of temporal lobe epilepsy. Epilepsy Research. 102 (3), 153-159 (2012).
- Jonasson, Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neuroscience and Biobehavioral Reviews. 28 (8), 811-825 (2005).
- Prendergast, B. J., Onishi, K. G., Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews. 40, 1-5 (2014).
- Ingham, N. J., Pearson, S., Steel, K. P. Using the Auditory Brainstem Response (ABR) to Determine Sensitivity of Hearing in Mutant Mice. Current Protocols in Mouse Biology. 1 (2), 279-287 (2011).
- . SigGenRZ Manual Available from: https://www.tdt.com/files/manuals/SigGenRZ_Manual.pdf (2012)
- Bogaerts, S., Clements, J. D., Sullivan, J. M., Oleskevich, S. Automated threshold detection for auditory brainstem responses: comparison with visual estimation in a stem cell transplantation study. BMC Neuroscience. 10, 104 (2009).
- Probst, F. J., et al. A point mutation in the gene for asparagine-linked glycosylation 10B (Alg10b) causes nonsyndromic hearing impairment in mice (Mus musculus). PLOS ONE. 8 (11), e80408 (2013).
- Alvarado, J. C., Fuentes-Santamaria, V., Gabaldon-Ull, M. C., Blanco, J. L., Juiz, J. M. Wistar rats: a forgotten model of age-related hearing loss. Frontiers in Aging Neuroscience. 6, 29 (2014).
- Du, P., Kibbe, W. A., Lin, S. M. Improved peak detection in mass spectrum by incorporating continuous wavelet transform-based pattern matching. Bioinformatics. 22 (17), 2059-2065 (2006).
- Daubechies, I. . Ten lectures on wavelets. , (1992).
- Pearson, J. D., et al. Gender differences in a longitudinal study of age-associated hearing loss. Journal of the Acoustical Society of America. 97 (2), 1196-1205 (1995).
- Murphy, M. P., Gates, G. A. Hearing Loss: Does Gender Play a Role?. Medscape Womens Health. 2 (10), 2 (1997).
- Henry, K. R. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hearing Research. 190 (1-2), 141-148 (2004).
- Ison, J. R., Allen, P. D., O’Neill, W. E. Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. Journal of the Association for Research in Otolaryngology. 8 (4), 539-550 (2007).
- Zheng, Q. Y., Johnson, K. R., Erway, L. C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Research. 130 (1-2), 94-107 (1999).
- Zhou, X., Jen, P. H., Seburn, K. L., Frankel, W. N., Zheng, Q. Y. Auditory brainstem responses in 10 inbred strains of mice. Brain Research. 1091 (1), 16-26 (2006).
- Lundt, A., et al. Cav3.2 T-Type Calcium Channels Are Physiologically Mandatory For The Auditory System. Neuroscience. , (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved