Method Article
Structured Motor Rehabilitation After Selective Nerve Transfers
In This Article
Summary
Here, we present a protocol for the motor rehabilitation of patients with severe nerve injuries and selective nerve transfer surgery. It aims at restoring the motor function proposing several stages in patient education, early-stage therapy after surgery and interventions for rehabilitation after successful re-innervation of the nerve’s target.
Abstract
After severe nerve injuries, selective nerve transfers provide an opportunity to restore motor and sensory function. Functional recovery depends both on the successful re-innervation of the targets in the periphery and on the motor re-learning process entailing cortical plasticity. While there is an increasing number of methods to improve rehabilitation, their routine implementation in a clinical setting remains a challenge due to their complexity and long duration. Therefore, recommendations for rehabilitation strategies are presented with the aim of guiding medical doctors and therapists through the long-lasting rehabilitation process and providing step-by-step instructions for supporting motor re-learning.
Directly after nerve transfer surgery, no motor function is present, and therapy should focus on promoting activity in the sensory-motor cortex areas of the paralyzed body part. After about two to six months (depending on the severity and modality of injury, the distance of nerve regeneration and many other factors), the first motor activity can be detected via electromyography (EMG). Within this phase of rehabilitation, multimodal feedback is used to re-learn the motor function. This is especially critical after nerve transfers, as muscle activation patterns change due to the altered neural connection. Finally, muscle strength should be sufficient to overcome gravity/resistance of antagonistic muscles and joint stiffness, and more functional tasks can be implemented in rehabilitation.
Introduction
Selective nerve transfers provide an opportunity for restoring the motor function after nerve injuries when recovery by the use of neurolysis, nerve repair, or nerve grafting cannot be expected1,2. Possible indications for nerve transfers are severe distal nerve injuries, avulsion-type injuries, the lack of available nerve roots for grafting, the extensive scarring at the injury site and delayed reconstruction3,4. Following motor nerve injury, reconstruction is time-critical as degeneration of muscle tissue and motor end plates only allow for the successful muscle re-innervation within 1-2 years after injury5,6. Here, nerve transfers provide the advantage of a relatively short re-innervation time after surgery, as they allow nerve coaptation close to the target. This procedure, also known as neurotization, involves the surgical redirection of an intact nerve (donor nerve) to the distal part of the recipient nerve. As this connection is distal to the damaged site of the recipient nerve, it allows bypassing the injured nerve segment7.
As neural pathways are altered after nerve transfer surgery, patients cannot be treated with standard post-operative therapy protocols otherwise used after direct nerve repair8,9. While donor axons grow into the new target, they take over a function they did not have before while cortically still being connected to their original function. As an example, the Oberlin ulnar nerve transfer is used to restore elbow flexion after irreparable damage to the upper trunk or nerve roots C5 and C61. As shown in Figure 1, it involves transferring one or more ulnar nerve fascicles to the musculocutaneous motor branch of the biceps muscle10. However, after the successful re-innervation, these fascicles of the ulnar nerve are cortically still connected to their previous function of finger flexion and/or ulnar abduction and flexion of the wrist. On a functional level this implies that at the beginning of the rehabilitation, the patient needs to focus on the previous nerve function (hand closing) in order to activate and strengthen the recipient muscle (biceps contraction). This approach is also known as “donor activation focused rehabilitation approach”9.
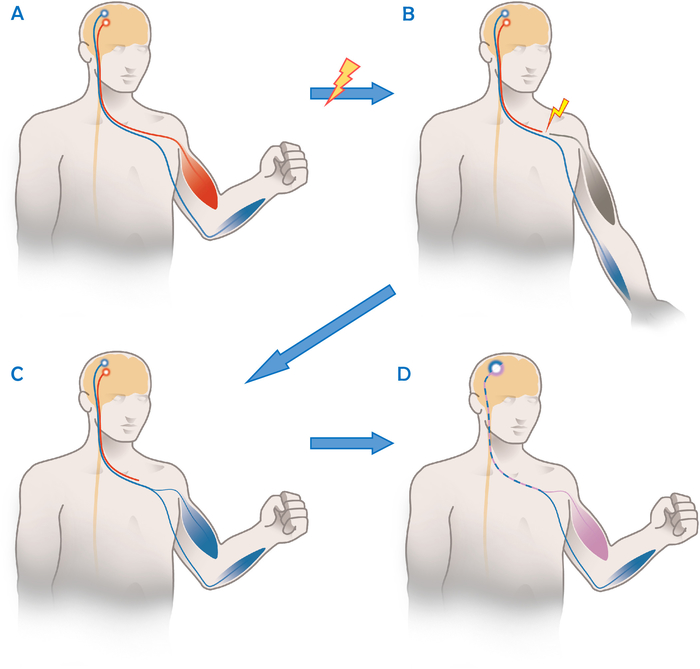
Figure 1: Schematic illustration of the functional principle of an ulnar to musculocutaneous nerve transfer. (A) In a healthy person, there is a clear separation between activity in the motor cortex for functions of different nerves/joints as here the musculocutaneous nerve (red) and the ulnar nerve (blue). (B) After an injury of the musculocutaneous nerve, the biceps muscle cannot be activated, while the uninjured ulnar nerve (in blue) still functions. (C). After the Oberlin’s nerve transfer and re-innervation, fascicles of the ulnar nerve control the biceps muscles as well as all other muscles anatomically innervated by the ulnar nerve. Before cortical reorganization occurs, both muscles are activated together as there is no cortical separation between these nerve fibers (in blue). (D) With successful rehabilitation, the patient has learned to use certain cortical axons for “normal” ulnar nerve functions (in blue), while others (in purple) are now controlling the biceps muscle. This allows independent movement of both muscle groups. Please click here to view a larger version of this figure.
While comprehension of this concept is the foundation of successful rehabilitation, re-learning of new motor patterns can be challenging for patients and clinicians. This is due to the long duration of rehabilitation, the complexity of nerve regeneration and re-innervation and the limited amount of directly observable muscular activity during early re-innervation8. Apart from the changes in the peripheral nervous system, there is an increasing awareness among surgeons and therapists for the relevance of changes in the central nervous system (CNS), i.e., re-organization of the hand motor and sensory cortical areas occurring as a consequence to denervation11. When neural input to the CNS is deprived, the associated cortical area diminishes to a certain extent at the expense of adjacent areas12. Restoration of function, therefore, depends on the central recovery of its representation in the brain. Within the last years, the use of biofeedback methods8 and approaches to support cortical re-organization13,14,15 has led to extended possibilities in rehabilitation after nerve transfers. However, due to the complexity of post-surgical therapy, it is important to provide the right interventions at the right time13.
Therefore, the aim of this structured protocol for rehabilitation after selective nerve transfers is to provide a feasible and holistic approach to support motor recovery. It is based on current recommendations and the authors’ experience with incorporating it in a clinical setting. The protocol is meant to guide medical doctors, occupational and physical therapists as well as other health professionals through the long-lasting rehabilitation process.
This structured protocol for motor rehabilitation was evaluated in a feasibility study8 in five patients with brachial plexus injuries as shown in Table 1. All of them received several nerve transfers (some in combination with nerve grafts) to restore upper extremity function. Therefore, for the sake of clarity, when describing specific interventions in this protocol, they refer to the upper limb. In detail, we take the Oberlin ulnar nerve transfer10 as an example, which was performed in patients 1-3. For this, we refer to parts of the ulnar nerve as being the donor nerve and the musculocutaneous nerve being the recipient nerve. Thus, the biceps and brachialis muscles are the recipient muscles being re-innervated by parts of the ulnar nerve. Functionally, this means that following a donor activation focused approach9, movements associated with ulnar nerve activity (such as hand closing or ulnar abduction of the wrist) are used for the activation of the biceps muscle directly after re-innervation. However, exercises based on this approach can be performed in other body parts as well. If special considerations are necessary to implement this in other body parts (e.g., the lower extremity), this is pointed out within the protocol.
Independent from the body part affected, therapy sessions should not exceed 30 min as muscles become easily fatigued shortly after re-innervation8 and successful training requires a patient’s full commitment and focus.
Protocol
The study was approved by the local research ethics committee (number: 1009/2014) and carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in this study.
1. Patient Education
- Despite any given previous information to the patient, use the first post-surgical consultation/therapy session to thoroughly explain the type of injury, as well as the performed surgery in detail.
- Visualize the nerve transfers, that were performed, on a scheme or print-out from an anatomy figure.
- Explain how the altered neural pathway initially requires thinking of a nerve’s original movement pattern.
- Give the patient a rough rehabilitation plan and an idea of what outcomes might be realistic at what point in time.
- If the patient suffers from the negative consequences of the injury on a psychological level16,17 or needs support in coping with stress or pain, contact a psychologist.
- Ask the patient to explain the impact of the nerve transfers in their own words to find out how they understood the concept.
- If necessary, repeat certain explanations and answer open questions.
- If the rehabilitation institution has a leaflet with the most important facts, hand this over to the patient (see Supplementary File for an example).
- Discuss a home program with the patient.
- Explain that a high frequency of training is important for good outcomes, and thus home exercises are an integral part of rehabilitation.
- Discuss with the patient how he/she thinks this can be best approached. Thus, empower the patient to assume responsibility for his/her own rehabilitation.
- Hand out the discussed home exercise program in a written form. Make sure it only contains exercises that were previously performed within a therapy session.
- In order to preserve adherence over time, regularly ask the patient how he/she feels about the home program and discuss how it should be altered to be feasible and meaningful to the patient.
2. Enhancing Cortical Re-presentation of the Denervated Body Part
NOTE: The following rehabilitation techniques promote activation of the denervated motor and sensory cortical areas to regain cortical representation of the paralyzed body part. During this phase no active muscle contraction is possible.
- Follow an approach for lateralization training (left/right discrimination) as described by Mosely et al.18.
- Prepare cards showing left and right extremities in a random order (upper extremity, if the upper extremity is affected and lower extremity for lower extremity nerve injuries). Show them to the patient in a random order.
- Ask the patient if a left or a right extremity is shown. While speed of approximately 2 s/card is normal18, give the patient at least 15 s to answer, if needed.
- Give the patient feedback, and if necessary, time to understand why the answer was wrong.
- Do this for 5 to 10 min to avoid fatigue. Ask the patient to do this at home as well, twice a day for 5-10 min.
- Instruct the patient to imagine movements of the paralyzed body part, although no motor output is expected.
- Make sure that the patient is in a calm environment without any distraction.
- Ask the patient which movements of the paralyzed extremity are easy to imagine.
- Instruct the patient to imagine these movements for about 5 min with the exact timing depending on the patient’s ability to fully concentrate on these imagined movements.
- Within the treatment process, instruct the patient to imagine more complex motions as well.
- As a home exercise, ask the patient to imagine these movements 5 to 10 min, twice a day.
- Use the mirror therapy to create the illusion of active movement of the paralyzed part19,20.
- Place a standing mirror or a mirror box in front of the therapist and the patient. Place it on a desk for the upper extremity or on the floor for the lower extremity.
- Explain that mirror therapy works by making use of the reflection of the sound side to create the image of the simultaneous movement of the sound side and denervated extremity19,21. Shortly demonstrate this with the therapist’s own upper or lower extremity.
- Place the mirror medially in front of the patient in a way that he/she sees the reflection of the sound side exactly where the injured extremity is expected. Make sure that the whole injured extremity is covered by the mirror (box), i.e., it cannot be seen by the patient.
- Ask him/her which movements he/she can easily imagine. Instruct the patient to perform these movements with the sound side while looking at the mirror. Start with slow movements.
- Instruct the patient to move both sides for 5 to 10 min. Explain, that the injured side will not move, but that it is still important to generate the illusion of simultaneous movement of both sides.
- Within the treatment process, encourage the patient to also perform movements that he/she cannot imagine easily to gradually increase the difficulty.
- As a home exercise, ask the patient to perform/imagine these movements 5 to 10 min, twice a day.
NOTE: Together with the other exercises to enhance cortical reorganization, this accounts for about 20 min of the home program, twice a day. Ask the patient if this is feasible. Otherwise, choose one or two of these interventions based on the patient’s preferences and reduce the training time to a manageable amount.
- As there is no active motion expected within the first months after surgery, make sure that the range of motion (ROM) is preserved in all joints.
- Let the patient actively move all joints.
- Instruct the patient to perform this every day by him/herself.
- Additionally, in paralyzed hands or ankles use splints or orthoses to stabilize the joints in a position that avoids contractures of joints, ligaments, and tendons (as the intrinsic plus position for the hand22). If necessary, fabricate a hand splint or make sure that the patients get a well-fitted device. In patients with an unstable shoulder and/or no elbow flexion use a sling15.
- Depending on the patient’s needs, include exercises for body symmetry, trunk stability, and posture. Especially, if hand function is severely impaired, include training of one-handed activities and provide the patient with assistive devices.
3. Motor Activation Using a Donor Side Approach
- Start this part of the rehabilitation as soon as the first volitional contraction of the re-innervated muscle can be detected, which can usually be expected within 3-5 months after surgery (see Table 2).
- Set up a system for surface EMG biofeedback by unpacking it on a table, plugging in all cables and pressing the power button. This can be a stand-alone device, or one connected to a computer. If a computer is used, connect the device with the computer and start the appropriate software.
- Prepare the patient’s skin to reduce impedance23. Do so by carefully shaving the respective body part and/or by gently removing dead skin cells with a peeling gel and/or a wet paper towel. Shortly explain the functionality of the system to the patient.
- Ask the patient to think of movements that the donor nerves were originally responsible for (e.g., hand closing if the ulnar nerve was used) and palpate the recipient muscle.
- Place a surface EMG electrode on the exact position, where muscle contraction can be palpated. While surface EMG may be detected with wet and dry electrodes, in this experiment dry electrodes are preferred for testing as these can be easily moved on the skin to alter electrode position. Even if no movement can be palpated, check for the EMG activity regularly within the first 3-6 months after surgery.
NOTE: The re-innervation can be confirmed, if the signal amplitude during activation is repeatedly 2-3 times higher than background noise during relaxation8. - If this cannot be confirmed, slightly change the position of the electrode and try other motor commands related to the donor nerve (e.g., ulnar abduction or flexion of the wrist, if the ulnar nerve was used as a donor). Otherwise, continue with the interventions for cortical activation and test again after a few weeks.
- Train the activation of the newly re-innervated muscles with sEMG biofeedback.
- As the first step of muscle activation training, educate the patient on the function of sEMG biofeedback and explain the principles of the donor activation approach.
- Switch on the biofeedback system and place the surface EMG electrode on the patient’s skin above the muscle to display the patient’s muscle activation.
- Make sure that the patient is comfortably seated and instruct the patient to think of movement patterns related to the donor nerve while picking up sEMG signals from the recipient muscle. If a system with the possibility to adjust signal gains is used, set it up in a way that the signal amplitude is high enough to be easily observed. In the beginning, this usually requires a high amplification.
- As soon as the patient can repeatably activate the muscle, ask him/her to fully relax after muscle activation, which corresponds to EMG amplitudes close to zero. Full relaxation is often hard to achieve for the patient and can take some time. Ask the patient to repeatedly activate the muscle and fully relax it.
- Try different movement cues and electrode positions in order to find the highest amplitude. After finding a good combination, maintain it the rest of the session.
- Provide the patient with a structured home exercise program including the amount of training per week (10-20 min of concentrated training per day is recommended) and exact instructions of what to train. If it is possible for the patient to use a device for sEMG biofeedback at home, encourage this8. Update the home exercise program regularly.
- As soon as the patient feels confident with the sEMG setup, introduce motor commands including both the activation of the donor nerve and the actual function of the recipient muscle. For a patient with an Oberlin’s ulnar nerve transfer to the biceps muscle, this means thinking of hand closing and elbow flexion at the same time.
CAUTION: In patients where a nerve branch from an antagonistic muscle was transferred, do only focus on the donor nerve function and omit this step. - Train muscle activation with and without sEMG biofeedback until muscle strength is sufficient to overcome gravity or resistance of antagonistic muscles. Additionally, repeat the interventions for activation of the motor cortex.
4. Re-learning the Original Movement Pattern
- As soon as the muscle is strong enough to overcome gravity or the resistance of antagonistic muscles and joint stiffness, focus therapy on re-learning the original movement pattern of the recipient nerve. This means that a patient after an Oberlin’s ulnar nerve transfer finally needs to learn how to flex the elbow without any movement of the hand and conversely, move the hand without flexion of the elbow.
- Encourage the patient to slightly activate the recipient muscle without the movement in the muscles originally innervated by the donor nerve.
- Support this by using sEMG biofeedback with two channels. Place one bipolar electrode on the skin above the re-innervated muscle and put the other one on the skin above the original donor nerve muscle. This allows the patient to simultaneously see the activation of both muscles. Encourage the patient to activate the recipient muscle and ensure that the donor muscle is relaxed with a low EMG signal amplitude.
- Let the patient know that signal separation is usually easier with slight muscle activation and that unwanted co-contraction of both muscles is common at the beginning of training.
- Using the same sEMG setup, ask the patient to activate the donor muscle without the activation of the re-innervated muscle and monitor for desirable/undesirable strategies resulting in better/worse separation of signals. Encourage strategies that support signal separation.
- If both signals can be separated with slight muscle contractions, ask the patient to perform stronger contractions.
- As soon as good signal separation while using sEMG biofeedback can be observed, ask the patient to perform separated “donor” and “recipient” movements without feedback.
- As this phase is cognitively demanding and repetition is of great importance for motor re-learning, make sure that the patient has a suitable home exercise program. Again, encourage the use of sEMG biofeedback devices at home, if possible.
- With increased motor function, encourage the patient to do more complex tasks including increased muscle force or improved precision. Also start “classic” strengthening exercises, if necessary.
- Finally, focus on activities of daily living and those needed in the patient’s home, work environment and when performing sports.
- In lower limb nerve transfers, start gait training with the focus on avoiding undesired compensatory movements.
- Ask the patient to walk along a corridor and analyze the gait based on the principles of observational gait analysis24,25.
- Define deviations from the physiological gait pattern and analyze them with respect to the origin (e.g., which muscle might be weak) and the connection between each other (e.g., how hip kinematics affects knee kinematics and vice versa). If necessary, for clarification, conduct additional tests (e.g., for muscle strength or joint mobility).
- Develop a treatment plan based on your findings24,25.
- Evaluate the interventions while the patient is doing them, as well as the therapy progress over time. If necessary, conduct another gait analysis and/or change interventions.
- See the patient three, six and twelve months after discharge from rehabilitation to find out about the long-term therapy success and patient satisfaction. If necessary and requested by the patient provide further training sessions.
- Evaluate, if the functional goal, that was discussed with the patient before surgery/at the beginning of rehabilitation could be reached.
NOTE: For some patients, this might be fully functional recovery, while for others the return of minimal function might be sufficient.- Ask the patient, if he/she is satisfied with the rehabilitation outcome and make clear that this is very subjective and is not necessarily reflected by any scores in outcome assessment instruments.
- If the patient is unsatisfied with the outcome, inform the patient about further (surgical) strategies to enhance function, as well as the possibility of using functional orthoses to compensate limited muscle strength.
Results
The described rehabilitation protocol was implemented in a clinical setting at the Medical University of Vienna and its feasibility was assessed in a previous study8.
As reported in our previous publication8, five patients participated in the trial to evaluate the feasibility and outcomes of such a program for motor rehabilitation after complex peripheral nerve injuries. Patient characteristics including injury and performed surgical reconstruction can be found in Table 1. All of the included patients suffered severe brachial plexus injuries. Thus, motor recovery without surgical intervention was deemed unlikely and direct nerve suture was not possible in any of the cases. The performed nerve transfers were chosen depending on the intact anatomy, and where possible, nerve transfers from agonistic muscles were performed. This was done to reduce the cognitive load during motor re-learning.
In order to evaluate the motor outcomes, the patients’ muscle strength was evaluated prior to reconstructive surgery and after discharge from rehabilitation using the British Medical Research Council (BMRC) scale26.
The results presented in Table 2 show that all patients had an improved shoulder and elbow function after rehabilitation, allowing them to flex the arm against gravity. This is in line with earlier research, reporting that a majority of patients regain useful shoulder and elbow function after selective nerve transfers and rehabiliation3,27,28. However, two of the patients with an Oberlin’s ulnar nerve transfer included in this study, regained full elbow flexion strength (M5), which is better than described by Bertelli and Ghizoni (2004)29 who used the same surgical method. However, Ray et al. (2011)28 could also show full recovery of elbow function in some of the patients treated in their center. Therefore, the presented motor outcomes are similar or slightly better than those described in the literature. This indicates that this protocol contributes to good outcomes in proximal muscles, where re-innervation of the muscles is likely.
However, in more distal parts of the body, the full function could not be regained for all patients, which is in line with other research3,30. While we believe that motor re-education using a structured training protocol may facilitate motor rehabilitation by the central recovery of the hand’s representation in the brain, it has limited influence on the peripheral processes needed for the re-innervation of muscles after nerve transfer surgery. Thus, the authors propose the use of this protocol, if peripheral nerve re-innervation is expected, but do not believe it to promote nerve regeneration at the peripheral level.
| Case nr. | Sex, Age (years) | Type of Accident | Type of Lesion | Reconstructive surgeries for restoration of upper limb function | ||||
| 1 | m, 68 | Motorcycle accident | Polytrauma; Global brachial plexopathy | Nerve grafts to bridge defect of MCN; thoracodorsal nerve grafts to bridge defect of axillary nerve; nerve grafts for posterior trunk reconstruction; Oberlin’s ulnar nerve transfer to MCN motor branch to the short head of the biceps | ||||
| 2 | m, 56 | Bicycle accident | Nerve root avulsion of C5-C6 | Oberlin’s ulnar nerve transfer to MCN motor branch for restoration of biceps function; transfer of radial triceps motor branch to axillary nerve | ||||
| 3 | m, 62 | Bicycle accident | Extensive damage to superior trunk of the BP; traction injury of C7 | XI-to-suprascapular nerve transfer; end-to-end transfer of phrenic nerve to C7; transfer of ulnar nerve fascicle to biceps motor branch of MCN; transfer of median nerve fascicle to brachialis motor branch of MCN; transfer of radial nerve fascicle to axillary nerve | ||||
| 4 | f, 22 | Car accident | Nerve root avulsion of C7; damage to C8 and T1 | Nerve grafts from C5 and C6 to MCN, median and radial nerve; nerve grafts from C8 to median, radial and ulnar nerve; nerve grafts from T1 to ulnar nerve | ||||
| 5 | f, 43 | Minor trauma years after OBPL | Traction injury of superior and medial trunk of the BP | Nerve grafts to bridge defect of C5, C6 and C7 to restore elbow function and shoulder stability; transfer of median nerve fascicle to brachial motor branch of MCN | ||||
Table 1: Patient characteristics. Please note the following abbreviations: BP = brachial plexus; MCN = musculocutaneous nerve; OBPL = obstetrical brachial plexus lesion; OP = operation; XI = spinal accessory nerve. This table is adapted from Sturma et al. (2018)8.
| Case nr. | Upper limb function including BMRC grades at baseline | Upper limb function including BMRC grades at follow-up | Time between nerve transfer surgery and first volitional sEMG activity | No. of Therapy Sessions in total (30 min each) | ||||
| 1 | Deltoid muscle: 0 | Deltoid muscle: 2 | 5 months | 25 | ||||
| Elbow flexion: 0 | Elbow flexion: 3 | |||||||
| Triceps muscle: 0 | Triceps muscle: 2 | |||||||
| No active hand function | Wrist extension: 1 | |||||||
| Finger extension: 2 | ||||||||
| 2 | Elbow flexion: 1 | Elbow flexion: 5 | 4 months | 22 | ||||
| Deltoid muscle: 2- | Deltoid muscle: 5 | |||||||
| 3 | Elbow flexion: 0 | Elbow flexion: 5 | 3 months | 30 | ||||
| Deltoid muscle: 0 | Deltoid muscle: 4 | |||||||
| Triceps muscle: 3 | Triceps muscle: 5 | |||||||
| Wrist extension: 3+ | Wrist extension: 5 | |||||||
| Finger flexion: 3+ | Finger flexion: 5 | |||||||
| 4 | Elbow flexion: 0 | Elbow flexion: 3+ | 5 months | 20 | ||||
| Triceps muscle: 0 | Triceps muscle: 2 | |||||||
| No active hand function | Wrist flexion: 3 | |||||||
| Finger flexion (ulnar FDP part): 3 | ||||||||
| 5 | Elbow flexion: 0 | Elbow flexion: 3 | 4 months | 18 | ||||
| Deltoid muscle: 2 | Deltoid muscle: 2 | |||||||
| Triceps muscle: 3+ | Triceps muscle: 4 | |||||||
| Mean (±SD) | 4.2 ± 0.75 months | 23 ± 4.20 | ||||||
Table 2: Motor outcomes of the rehabilitation protocol. There was no functional impairment of the muscles not included in the table. In all patients, shoulder and elbow function were impaired at baseline and improved to follow-up. Additionally, the time between surgery and first volitional sEMG activity, as well as the number of therapy sessions for each patient are presented. This table is adapted from Sturma et al. (2018)8.
Supplementary File. Please click here to download the file.
Discussion
Recently, nerve transfers have been increasingly used to restore function after severe proximal nerve injuries with promising outcomes1,4,31,32. However, while there is a consensus that structured training programs are necessary to promote beneficial neuroplastic changes33,34,35, there is no structured protocol available to describe motor rehabilitation approaches after nerve transfers step-by-step. Therefore, the aim of the presented protocol was to provide detailed instructions for post-surgical rehabilitation to embrace cortical changes and enhance surgical outcomes. In contrast to other protocols9,36, visualization of muscular activity via surface EMG biofeedback is a key element in the presented protocol.
Within therapy, patient education is a critical step as the patient needs to understand the rather complex surgical procedure and be educated on activities improving the health status in order to be actively involved in the long rehabilitation process8,13,37. There is broad agreement that repetition is fundamental and daily home exercises are needed to reinforce a well-established cortical representation of the hand8,34,38,39. Apart from pure patient information, the authors strongly recommend a patient-centered approach for rehabilitation. This additionally involves treating the patient as a unique person, the involvement of the patient in care, good clinician-patient communication and empowering the patient. In medical rehabilitation, this approach positively influences patient satisfaction and outcomes40. Regarding the motor rehabilitation itself, it is recommended to start interventions before re-innervation of the muscles and to follow a donor activation focused approach9. To ensure that muscular activity is detected as early as possible, EMG biofeedback devices can be used. While the authors are aware that EMG biofeedback devices are not yet clinical standard, their use is highly recommended as they allow to start early active motor rehabilitation and provide valuable feedback on newly re-innervated muscles8.
The principles described within this protocol can be applied for different types of nerve transfers, although modifications within the protocol might be necessary. While motor re-learning is relatively easy if synergistic muscles/nerves were used, the use of antagonistic muscles/nerves requires a longer rehabilitation time and the use of biofeedback might be of even greater importance3,8. Especially in those cases where a higher amount of repetitions is needed, future protocols might also include serious games to maintain patient motivation41.
As the timing of nerve regeneration and the amount of recovery hugely depends on the injury and surgical interventions, there is no strict timeline for rehabilitation. Instead, the therapist is asked to proceed depending on the signs of motor recovery as stated in the protocol. In the same way, it is important to note that the success of nerve transfer surgery is based on many factors including type and severity of the injury, the surgeon’s skills, and expertise as well as the patient’s age, health status, cognition and motivation8,13,42,43. While rehabilitation is a main pillar for regaining function after severe nerve injuries, even the best program for motor re-education cannot improve function, if there are inadequate peripheral nerve regeneration and muscle re-innervation. Thus, the authors strongly recommend seeing the patients regularly together within a multidisciplinary team to be able to discuss if recovery goes as expected or if any additional medical interventions are necessary. However, especially after severe injuries such as C8 and Th1 nerve root avulsions, realistic outcomes might not include full recovery of extremity function3,30. In these cases, the clinical team needs to communicate this to the patient as soon as a realistic prognosis can be stated (approximately one year after the nerve transfers). At this point, further possibilities in rehabilitation, assistive devices or surgical interventions (as tendon transfers) need to be discussed. In cases, where absolutely no hand function returns, replacing the functionless limb with a prosthetic device can be considered as an option as well44,45. This is, however, only recommended as a last resort and after in-depth physical and psychological assessment46.
While the focus of peripheral nerve surgery usually lies on the reconstruction of motor function, sensory nerve transfers are sometimes used to restore the sensation in the hand after severe median or ulnar nerve injury4,47. Similar to motor nerve transfers, this creates altered sensory neural pathways and results in sensations that are felt as if they were originating from the previous innervation area of the donor nerve. Even if no sensory nerve transfers were performed, there can still be changed/reduced sensation either due to the injury itself27 or due to donor-side morbidity48. In these cases, timely re-education can help to improve the sensory function49, and reduce unwanted hyper-sensitivity and pain that often occurs after such injuries. To ensure good motor and sensory function, the authors strongly recommend complementing motor re-education with tailored therapy approaches to promote re-organization in the corresponding sensory cortex as well39,50,51. Regarding sensory re-education, it is recommended to start interventions before re-innervation of the skin49,52,53. This can include substitution of sensation by other senses as vision53 or auditory feedback54, as well as making use of the overlap of sensory innervation areas27,52. As soon as the patient has regained a certain amount of sensitivity, tactile gnosis and object recognition can be trained, while maintaining a high amount of sensory input34. Typical materials that can be used for this, include self-made plates with different surfaces to be recognized with closed eyes (see Figure 2) or a box filled with beans/lentils/rice (see Figure 3).
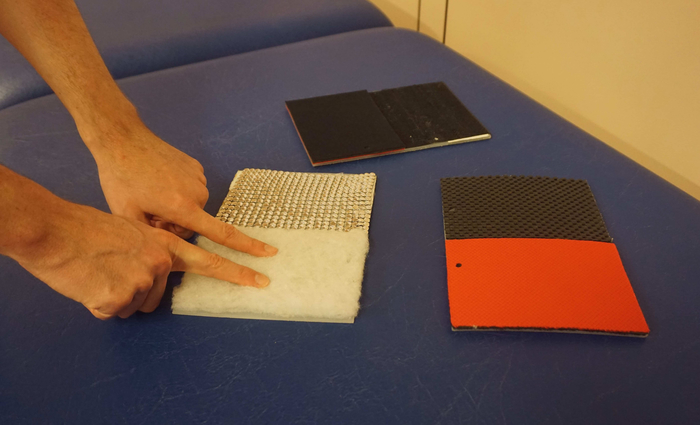
Figure 2: Different surfaces can be used to support regaining of sensibility. Usually, the patient is asked to touch these with both hands first, while he/she might try afterwards to recognize the different surfaces without vision using only the hand with limited sensibility. Please click here to view a larger version of this figure.
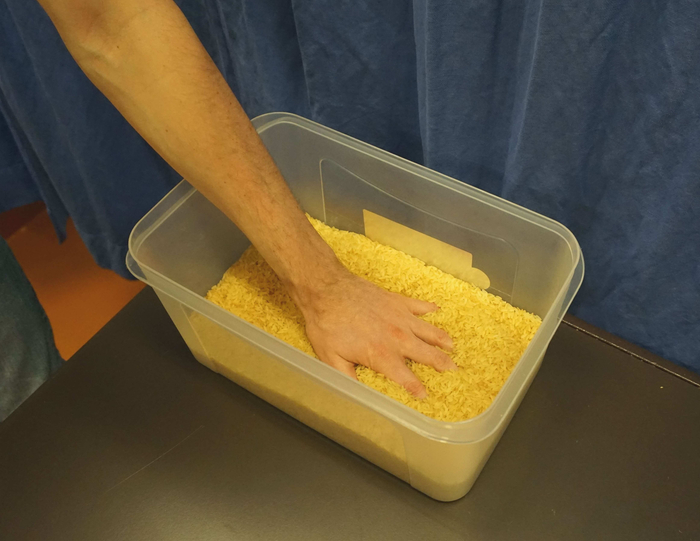
Figure 3: A box filled with rice for sensory re-education of the hand. In therapy, the patient might put his/her hand with reduced sensitivity carefully in this box and slowly move the hand. To focus the patient’s attention, the therapist can put some small objects (e.g., wooden blocks or paper clips) in this box and ask to find them without visual control. Please click here to view a larger version of this figure.
However, in both sensory and motor re-education, there is only limited evidence regarding the choice of interventions needed to promote good recovery34. This limits the validity of the proposed rehabilitation protocol, as for other protocols. While the described protocol was assessed within a feasibility study and motor outcomes were similar or slightly better than those reported in the literature8, this study was performed on a small sample size and without a control group. This makes it impossible to compare the outcomes, advantages, and disadvantages of this protocol with respect to previous ones. Further research needs to include controlled studies in order to compare the possible advantages of using surface EMG biofeedback to conventional approaches.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was funded by the Christian Doppler Research Foundation of the Austrian Council for Research and Technology Development and the Austrian Federal Ministry of Science, Research and Economy. We thank Petra Gettinger for her assistance in the preparation of filming and Aron Cserveny for the preparation of the illustrations included in the manuscript and the rehabilitation leaflet. Frontiers in Neuroscience granted permission for reproducing the data presented in the original paper.
Materials
| Name | Company | Catalog Number | Comments |
| EMG electrodes | Otto bock Healthcare, Duderstadt, Germany | electrodes 13E202 = 50 | The EMG electrodes used in this study were bipolar and included a ground and a 50 Hz filter. They were used with the Moby. |
| Folding Mirror Therapy Box (Arm/Foot/Ankle) | Reflex Pain Management Therapy Store | This box was used for mirror therapy. | |
| Myoboy | Otto bock Healthcare, Duderstadt, Germany | Myoboy | This EMG Biofeedback device that can be used as stand alone device or with a computer. While this device was used in the presented pilot study, other (cheaper) devices for sEMG biofeedback training are available as well. |
| Recognise[TM] Flash Cards | noigroup | If no self-made cards for left-right discrimination are used, these can be purchased from noigroup.com. There, a mobile app for training is available as well. |
References
- Rohde, R. S., Wolfe, S. W. Nerve transfers for adult traumatic brachial plexus palsy (brachial plexus nerve transfer). HSS Journal. 3 (1), 77-82 (2007).
- Ray, W. Z., Mackinnon, S. E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Experimental Neurology. 223 (1), 77-85 (2010).
- Tung, T. H., Mackinnon, S. E. Nerve Transfers: Indications, Techniques, and Outcomes. The Journal of Hand Surgery. 35 (2), 332-341 (2010).
- Isaacs, J., Cochran, A. R. Nerve transfers for peripheral nerve injury in the upper limb. Bone Joint Journal. 101 (2), 124-131 (2019).
- Terzis, J. K., Papakonstantinou, K. C. The surgical treatment of brachial plexus injuries in adults. Plastic and Reconstruction Surgery. 106 (5), (2000).
- Ray, W. Z., Mackinnon, S. E. Clinical Outcomes Following Median to Radial Nerve Transfers. The Journal of Hand Surgery. 36 (2), 201-208 (2011).
- Liu, Y., Lao, J., Gao, K., Gu, Y., Xin, Z. Outcome of nerve transfers for traumatic complete brachial plexus avulsion: results of 28 patients by DASH and NRS questionnaires. Journal of Hand Surgery European. 37 (5), 413-421 (2012).
- Sturma, A., Hruby, L. A., Prahm, C., Mayer, J. A., Aszmann, O. C. Rehabilitation of Upper Extremity Nerve Injuries Using Surface EMG Biofeedback: Protocols for Clinical Application. Frontiers in Neuroscience. 12 (906), (2018).
- Kahn, L. C., Moore, A. M. Donor Activation Focused Rehabilitation Approach: Maximizing Outcomes After Nerve Transfers. Hand Clinics. 32 (2), 263-277 (2016).
- Oberlin, C., et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. Journal of Hand Surgery American. 19 (2), 232-237 (1994).
- Karl, A., Birbaumer, N., Lutzenberger, W., Cohen, L. G., Flor, H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. Journal of Neurosciences. 21 (10), 3609-3618 (2001).
- Makin, T. R., Bensmaia, S. J. Stability of Sensory Topographies in Adult Cortex. Trends in Cognitive Science. 21 (3), 195-204 (2017).
- Novak, C. B., Lvonder Heyde, R. Rehabilitation of the upper extremity following nerve and tendon reconstruction: when and how. Seminars in Plastic Surgery. 29 (1), 73-80 (2015).
- Lundborg, G. Brain plasticity and hand surgery: an overview. Journal of Hand Surgery Bristish. 25 (3), 242-252 (2000).
- Novak, C. B. Rehabilitation Following Motor Nerve Transfers. Hand Clinics. 24 (4), 417-423 (2008).
- Miller, C., Peek, A. L., Power, D., Heneghan, N. R. Psychological consequences of traumatic upper limb peripheral nerve injury: A systematic review. Hand Therapy. 22 (1), 35-45 (2016).
- Bailey, R., Kaskutas, V., Fox, I., Baum, C. M., Mackinnon, S. E. Effect of Upper Extremity Nerve Damage on Activity Participation, Pain, Depression, and Quality of Life. The Journal of Hand Surgery. 34 (9), 1682-1688 (2009).
- Moseley, G. L. . The graded motor imagery handbook. , (2012).
- Ramachandran, V. S., Rogers-Ramachandran, D. Synaesthesia in phantom limbs induced with mirrors. Proceedings of the Royal Society of Biological Sciences. 263 (1369), 377-386 (1996).
- Rothgangel, A. S., Braun, S. M., Beurskens, A. J., Seitz, R. J., Wade, D. T. The clinical aspects of mirror therapy in rehabilitation. International Journal of Rehabilitation Research. 34 (1), 1-13 (2011).
- Ramachandran, V. S., Hirstein, W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 121 (Pt 9), 1603-1630 (1998).
- Hubatka, G., Meyer, V. E. Immobilization of the injured hand. Helvetica Chirurgica Acta. 47 (1-2), 81-84 (1980).
- Merletti, R., Parker, P. A. Electromyography: Physiology, Engineering, and Non-Invasive Applications. Wiley IEEE-Press Verlag. , (2004).
- Götz-Neumann, K. . Gehen verstehen. Ganganalyse in der Physiotherapie. , (2016).
- Perry, J., Burnfield, J. M. . Gait Analysis: Normal and Pathological Function. , (2010).
- James, M. A. Use of the Medical Research Council muscle strength grading system in the upper extremity. The Journal of Hand Surgery American. 32 (2), 154-156 (2007).
- Bertelli, J. A., Ghizoni, M. F., Loure Iro Chaves, D. P. Sensory disturbances and pain complaints after brachial plexus root injury: a prospective study involving 150 adult patients. Microsurgery. 31 (2), 93-97 (2011).
- Ray, W. Z., Pet, M. A., Yee, A., Mackinnon, S. E. Double fascicular nerve transfer to the biceps and brachialis muscles after brachial plexus injury: clinical outcomes in a series of 29 cases. Journal of Neurosurgery. 114 (6), 1520-1528 (2011).
- Bertelli, J. A., Ghizoni, M. F. Reconstruction of C5 and C6 brachial plexus avulsion injury by multiple nerve transfers: spinal accessory to suprascapular, ulnar fascicles to biceps branch, and triceps long or lateral head branch to axillary nerve. The Journal of Hand Surgery American. 29 (1), 131-139 (2004).
- Wong, A. H., Pianta, T. J., Mastella, D. J. Nerve transfers. Hand Clinics. 28 (4), 571-577 (2012).
- Colbert, S. H., Mackinnon, S. E. Nerve Transfers for Brachial Plexus Reconstruction. Nerve Transfers. 24 (4), 341-361 (2008).
- Brown, J. M., Mackinnon, S. E. Nerve Transfers in the Forearm and Hand. Nerve Transfers. 24 (4), 319-340 (2008).
- Beisteiner, R., et al. New type of cortical neuroplasticity after nerve repair in brachial plexus lesions. Archives in Neurology. 68 (11), 1467-1470 (2011).
- Novak, C. B., von der Heyde, R. L. Evidence and techniques in rehabilitation following nerve injuries. Hand Clinics. 29 (3), 383-392 (2013).
- Dahlin, L. B., Andersson, G., Backman, C., Svensson, H., Bjorkman, A. Rehabilitation, Using Guided Cerebral Plasticity, of a Brachial Plexus Injury Treated with Intercostal and Phrenic Nerve Transfers. Frontiers in Neurology. 8, 72 (2017).
- Hill, J., et al. . The stages of rehabilitation following motor nerve transfer surgery. , (2019).
- Vikstrom, P., Carlsson, I., Rosen, B., Bjorkman, A. Patients' views on early sensory relearning following nerve repair-a Q-methodology study. The Journal of Hand Therapy. 31 (4), 443-450 (2018).
- Anastakis, D. J., Malessy, M. J., Chen, R., Davis, K. D., Mikulis, D. Cortical plasticity following nerve transfer in the upper extremity. Hand Clinics. 24 (4), 425-444 (2008).
- Oud, T., Beelen, A., Eijffinger, E., Nollet, F. Sensory re-education after nerve injury of the upper limb: a systematic review. Clinical Rehabilitation. 21 (6), 483-494 (2007).
- Plewnia, A., Bengel, J., Korner, M. Patient-centeredness and its impact on patient satisfaction and treatment outcomes in medical rehabilitation. Patient Education Counselling. 99 (12), 2063-2070 (2016).
- Prahm, C., Kayali, F., Sturma, A., Aszmann, O. PlayBionic: Game-Based Interventions to Encourage Patient Engagement and Performance in Prosthetic Motor Rehabilitation. Physical Medicine & Rehabilitation. 10 (11), 1252-1260 (2018).
- Rosen, B., Lundborg, G., Dahlin, L. B., Holmberg, J., Karlson, B. Nerve repair: correlation of restitution of functional sensibility with specific cognitive capacities. Journal of Hand Surgery. 19 (4), 452-458 (1994).
- Lundborg, G., Rosen, B. Sensory relearning after nerve repair. Lancet. 358 (9284), 809-810 (2001).
- Aszmann, O. C., et al. Bionic reconstruction to restore hand function after brachial plexus injury: a case series of three patients. Lancet. 385 (9983), 2183-2189 (2015).
- Hruby, L. A., et al. Algorithm for bionic hand reconstruction in patients with global brachial plexopathies. Journal of Neurosurgery. , 1-9 (2017).
- Hruby, L. A., Pittermann, A., Sturma, A., Aszmann, O. C. The Vienna psychosocial assessment procedure for bionic reconstruction in patients with global brachial plexus injuries. PloS One. 13 (1), e0189592 (2018).
- Soldado, F., Bertelli, J. A., Ghizoni, M. F. High Median Nerve Injury: Motor and Sensory Nerve Transfers to Restore Function. Hand Clinics. 32 (2), 209-217 (2016).
- Li, X. M., et al. Donor-side morbidity after contralateral C-7 nerve transfer: results at a minimum of 6 months after surgery. Journal of Neurosurgery. 124 (5), 1434-1441 (2016).
- Rosen, B., Lundborg, G. Sensory re-education after nerve repair: aspects of timing. Handchirurgie Mikrochirurgie Plastiche Chirurgie. 36 (1), 8-12 (2004).
- Jerosch-Herold, C. Sensory relearning in peripheral nerve disorders of the hand: a web-based survey and delphi consensus method. Journal of Hand Therapy. 24 (4), 292-298 (2011).
- Rosén, B., Lundborg, G., Skirven, T. M., Osterman, A. L., Fedorczyk, J. M., Amadio, P. C. . Rehabilitation of the Hand and Upper Extremity. 6, (2011).
- Daniele, H. R., Aguado, L. Early compensatory sensory re-education. Journal of Reconstructive Microsurgery. 19 (2), 107-110 (2003).
- Rosen, B., et al. Enhanced early sensory outcome after nerve repair as a result of immediate post-operative re-learning: a randomized controlled trial. Journal of Hand Surgery European Volume. 40 (6), 598-606 (2015).
- Rosen, B., Lundborg, G. Early use of artificial sensibility to improve sensory recovery after repair of the median and ulnar nerve. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 37 (1), 54-57 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved