A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
LINE-1 Methylation Analysis in Mesenchymal Stem Cells Treated with Osteosarcoma-Derived Extracellular Vesicles
In This Article
Summary
Described here is the use of a methylation-specific probe amplification method to analyze methylation levels of LINE-1 elements in mesenchymal stem cells treated with osteosarcoma-derived extracellular vesicles. Ultracentrifugation, a popular procedure for separating extracellular vesicles from fetal bovine serum, is also demonstrated.
Abstract
Methylation-specific probe amplification (MSPA) is a simple and robust technique that can be used to detect relative differences in methylation levels of DNA samples. It is resourceful, requires small amounts of DNA, and takes around 4–5 h of hands-on work. In the presented technique, DNA samples are first denatured then hybridized to probes that target DNA at either methylated or reference sites as a control. Hybridized DNA is separated into parallel reactions, one undergoing only ligation and the other undergoing ligation followed by HhaI-mediated digestion at unmethylated GCGC sequences. The resultant DNA fragments are amplified by PCR and separated by capillary electrophoresis. Methylated GCGC sites are not digested by HhaI and produce peak signals, while unmethylated GCGC sites are digested and no peak signals are generated. Comparing the control-normalized peaks of digested and undigested versions of each sample provides the methylation dosage ratio of a DNA sample. Here, MSPA is used to detect the effects of osteosarcoma-derived extracellular vesicles (EVs) on the methylation status of long interspersed nuclear element-1 (LINE-1) in mesenchymal stem cells. LINE-1s are repetitive DNA elements that typically undergo hypomethylation in cancer and, in this capacity, may serve as a biomarker. Ultracentrifugation is also used as a cost-effective method to separate extracellular vesicles from biological fluids (i.e., when preparing EV-depleted fetal bovine serum [FBS] and isolating EVs from osteosarcoma conditioned media [differential centrifugation]). For methylation analysis, custom LINE-1 probes are designed to target three methylation sites in the LINE-1 promoter sequence and seven control sites. This protocol demonstrates the use of MSPA for LINE-1 methylation analysis and describes the preparation of EV-depleted FBS by ultracentrifugation.
Introduction
DNA methylation is a major epigenetic modification occurring in human cells. DNA methylation refers to the linkage of methyl groups to cytosine residues in CpG dinucleotides. Such dinucleotides are usually found in clusters (CpG islands) at the 5' region of genes1. In normal cells, most of these dinucleotides exist in an unmethylated state, which allows DNA transcription. Incidentally, many cancers are associated with hypermethylated CpG islands and transcriptomic silencing2, especially in tumor suppressor genes, which in turn contribute to various hallmarks of cancer3.
On the other hand, long interspersed nuclear elements-1 (LINE-1s or L1s) are repetitive, transposable DNA elements that normally have high levels of methylation at CpG islands. Methylation of LINE-1 prevents translocation and helps maintain genome integrity. In several types of cancer, LINE-1 is hypomethylated, resulting in activation and subsequent retrotransposition-mediated chromosomal instability4. LINE-1 accounts for nearly 17% of the human genome5, and its methylation status may serve as an indicator of global genomic methylation levels6. Global LINE-1 hypomethylation is considered to precede the transition of cells to a tumor phenotype7; therefore, it holds promise as a potential marker for early cancer onset.
Currently, there are several methods for methylation analysis, including pyrosequencing, methylation-specific PCR, microarrays, and chromatin immunoprecipitation1. The use of next-generation sequencing has also made it possible to incorporate genome-wide approaches to detection of DNA methylation. Many of these methods rely on bisulfite-treated DNA, in which unmethylated cytosines are converted to uracil and methylated cytosines remain unchanged. However, working with bisulfite-treated DNA has several pitfalls, such as incomplete conversions of unmethylated cytosines to uracil, biased amplification of sequences, and sequencing errors8.
In methylation-specific probe amplification (MSPA), probes composed of two oligonucleotides target DNA sequences containing a restriction site (GCGC) for the methylation-sensitive restriction enzyme HhaI9. After the probes hybridize to DNA, each sample is divided into two sets. Probes in the first set undergo ligation, while probes in the second set undergo ligation followed by HhaI-mediated digestion at unmethylated CGCG sites. Both sets of samples are then amplified by PCR, and the products are separated by capillary electrophoresis. Probes at unmethylated sites are digested by HhaI and are not amplified during PCR, resulting in no peak signals. By contrast, probes at methylated sites are protected from digestion and are therefore amplified during PCR, subsequently generating peak signals10.
MSPA has several advantages over alternative methods. First, it requires a low amount of DNA (50–100 ng) and is well-suited for analysis of DNA from formalin-fixed paraffin embedded samples10. It does not require bisulfite-treated DNA; in fact, it is unsuitable for DNA that is modified in this way. Many samples can be analyzed at the same time, and MSPA probes can be designed such that they target multiple genes or sequences simultaneously. Additionally, the probes are specific and sensitive for methylated DNA as the HhaI restriction site corresponds to a sequence that is typical of CpG islands10.
This study investigated the effects of osteosarcoma (OS)-derived extracellular vesicles (EVs) on LINE-1 methylation in adipose tissue-derived mesenchymal stem cells (AT-MSCs; Figure 1). EVs are nanoscale, membrane-bound vesicles secreted by most cell types. They carry proteins, lipids, mRNA, microRNA, and additional molecules from parent cells11,12. EVs mediate intercellular communication and play important roles in several pathophysiological conditions13,14. A recent study showed that cancer-derived EVs may transfer active LINE-1 to recipient cells15. It has been reported earlier that EVs from the HOS-143B cell line can alter the methylation status of LINE-1 in MSCs, in addition to other genetic effects16.
When growing cells for EV isolation, it is important to use EV-depleted fetal bovine serum [FBS] in the growth medium, since FBS-derived EVs may interfere with EVs from other sources and hamper the results17,18. Ultracentrifugation is one of the most common methods for depleting EVs from FBS. It is a relatively simple and cost-effective procedure compared to alternatives such as ultrafiltration and commercial EV-depleted FBS19. Here, the protocol also demonstrates how to prepare EV-depleted FBS by ultracentrifugation.
This article presents a detailed protocol for the aforementioned techniques, from isolation of EVs from an OS cell line to the methylation analysis of LINE-1 in OS-EV treated MSCs (Figure 1).
Protocol
This study was approved by the Ethics Committee of Helsinki and Uusimaa Hospital District (ethical approval D. No. 217/13/03/02/2015).
1. Preparation of EV-depleted FBS by ultracentrifugation
- Take FBS in (ultra)centrifuge tubes and place them in ultracentrifuge buckets. To ensure that the ultracentrifugation runs smoothly and safely, balance the buckets within 10 mg of each other.
- Load the buckets on a swinging rotor (type SW28, k-factor 246). Place the rotor in the ultracentrifuge and run at 100,000 x g for 19 h at 4 °C.
- Carefully collect the light-colored upper layer of the supernatant (approximately nine-tenths) and transfer to a 50 mL tube. Do not disturb or pipette the dark brown pellet, as it contains EVs from FBS.
- Pass the supernatant through a 0.22 µm filter into a new 50 mL tube.
- Add the filter-sterilized, EV-depleted FBS to cell culture media when growing cells for EV isolation.
2. Isolation of osteosarcoma-derived EVs
- Plate OS cells (HOS-143B cell line) in a T-175 flask with RPMI 1640 medium, supplemented with 10% normal FBS and 1% antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin). Place the flask in an incubator at 37 °C and 5% CO2.
- When the flask is 70%–80% confluent, wash the cells with phosphate-buffered saline (PBS) then grow them in media containing 10% EV-depleted FBS (hereafter referred to as EV-depleted media).
NOTE: 1 x 106 HOS-143B cells plated in a T175 flask reaches 70% confluency after approximately 60 h. - During the next 48 h, collect conditioned media from osteosarcoma cells after every 24 h and add fresh EV depleted media.
- Centrifuge the conditioned media at 2500 x g for 20 min at 4 °C to remove cells and cell debris. Transfer the supernatant to a new tube, leaving around 2 mL of media at the bottom.
NOTE: If not directly proceeding with EV isolation at this stage, the supernatant can be stored at -80 ᵒC. - Pour the supernatant into ultracentrifuge tubes and balance them as earlier. Centrifuge the tubes at 100,000 x g for 2 h at 4 °C.
- Carefully discard the supernatant, leaving around 1 mL at the bottom. Add around 20 mL of PBS (0.1 µm filtered) to the tube and pipette gently to wash and resuspend the EV pellet.
- Balance the tubes and perform another round of ultracentrifugation with the same settings.
- Carefully remove the supernatant and resuspend the EV pellet in 200 µL of PBS by gentle pipetting. Store the EVs in low binding tubes.
NOTE: EVs can be used straightaway or else stored in -80 °C until they are needed.
3. Characterization of OS-EVs
NOTE: Purified EVs can be characterized by western blotting (WB), nanoparticle tracking analysis (NTA), and transmission electron microscopy (TEM)16.
- Perform WB as per the standard protocol16 with EV markers CD63, TSG101, and Hsp70, and with calnexin as a negative control to indicate purity of the EV sample20.
- For NTA, first dilute the EV sample in 0.1 µm filtered Dulbecco's PBS to obtain (ideally) 30–100 particles per frame.
- Take around 500 µL of the sample into a 1 mL syringe and load it into the inlet port of the NTA instrument. Check that there are enough particles per frame, for accurate measurements.
- Open the NTA software and record five videos of 60 s duration, using camera level 13 at ambient temperature. During analysis, use detection threshold = 5 and gain = 10.
- Analyze EV samples by TEM as described previously21.
4. AT-MSC culture
NOTE: Human adipose tissue for mesenchymal stem cell isolation was provided as liposuction aspirate (Department of Plastic Surgery, Laser Tilkka Ltd., Finland). Written informed consent was taken from the lipoaspirate donors, who were undergoing elective liposuction procedures.
- Isolate AT-MSCs from liposuction aspirates using standard mechanical and enzymatic isolation methods22.
NOTE: MSCs from other sources (including commercial cell lines) may also be used. - Culture cells in DMEM/F-12 media supplemented with 10% FBS and 1% antibiotics.
5. Treatment of MSCs with OS-EVs
- Plate 15,000 AT-MSCs per well in a 24 well plate.
- After 24 h, remove the old media, wash cells with PBS, and change to EV-depleted media.
- Treat cells with OS-EVs (at a particle concentration of 1 x 106 EVs per cell) on Day 1 (24 h after cell adhesion), Day 3 (48 h after Day 1), and Day 5 (96 h after Day 1).
- Stop the OS-EV treatment for timepoint (TP) 0 samples on Day 1, TP 3 samples on Day 3 and TP 7 samples on Day 7 (48 h after Day 5).
NOTE: Other timepoint schedules as per the experimental plans can also be followed.
- Stop the OS-EV treatment for timepoint (TP) 0 samples on Day 1, TP 3 samples on Day 3 and TP 7 samples on Day 7 (48 h after Day 5).
- Extract DNA from the MSCs using an appropriate method. Include positive and negative control samples for the data analysis.
6. LINE-1 methylation assay
- Design the customized LINE-1 probe primers, as done previously by Pavicic et al.23. For methylation probes, select three sequences containing the HhaI restriction site within the promoter region of LINE-1. For control probes, select seven sequences lacking the HhaI restriction site from the rest of the LINE-1 sequence.
NOTE: The LINE-1 sequence is available at the GenBank database24 (L1.2, accession no. AH005269.2). Use the MSPA manufacturer's instructions for designing the probes25. - Dilute 70 ng of DNA sample in TE buffer to a 5 µL volume.
- Carry out subsequent thermocycling and PCR steps as mentioned in Table 1. Heat the samples for 10 min at 98 °C, then cool to 25 °C.
- Add 3 µL of probe hybridization mix to each sample and run the thermocycler to allow the probes to hybridize to the DNA.
- At room temperature (RT), add 13 µL of post-hybridization mix to each sample. Transfer 10 µL to a second tube.
- Place both sets of tubes in the thermocycler and incubate at 48 °C for at least 1 min.
- While samples are at 48 °C, add 10 µL of the ligation mix to the first set of tubes (undigested series) and 10 µL of the ligation-digestion mix to the second set of tubes (digested series). Run the next thermocycler program.
- Spin down the tubes and simultaneously set the thermocycler to 72 °C.
- Add 5 µL of polymerase mix to each tube and place the tubes in the thermocycler. Run the PCR program.
- While the PCR program is running, prepare a solution of 1 mL formamide containing 2.5 µL of size standard. Pipette 10 µL of this solution to each well of an optical 96 well plate (with barcode).
- After PCR, dilute the undigested and digested samples to 1:100 and 1:200 respectively in ultrapure water. Add 2 µL of diluted PCR product to the 96 well plate. Centrifuge the plate at 200 x g for 15–20 s to remove air bubbles.
- Carry out fragment analysis of samples by capillary electrophoresis.
NOTE: The plate should be stored at 4 °C in the dark until analysis.
7. Fragment data analysis
- Open the capillary electrophoresis results in an electropherogram analysis software.
- Under the Panel column, for one of the samples, choose MLPA from the menu. Click on the Panel header and press Ctrl+D to apply MLPA to all samples.
- In the same way, set the Analysis method to Microsatellite default for all samples.
- Select all samples and click on the Green play button to analyze the samples as per the chosen settings.
- Select all samples and click on the Graph button to visualize the probe peaks.
- Zoom in on the peak region for higher resolution of the individual probe peaks. Make sure that all 10 peaks corresponding to the probes from the LINE-1 probe-mix are labeled. Discard additional peaks (<95 bp and >160 bp).
- In the Genotypes tab, export the results in the comma-separated-values (CSV) format.
- Open the CSV file in a data analysis software and sort the data into columns.
- Label the three methylation site peaks based on their approximate sizes (L1-1m at 153 bp, L1-2m at 119 bp, L1-3m at 133 bp). The remaining seven peaks correspond to the control probes.
NOTE: Here, values of the L1-2m probe peaks are used, which have a size of 117 bp, since that region has been used in most LINE-1 methylation assays23. - For each sample (undigested and digested), calculate the sum peak area of all seven control peaks. Divide the peak area of each LINE-1 probe by this sum.
- For each DNA sample, divide the value of the digested sample by that of the undigested sample to obtain the methylation dosage ratio (DM) using the following equation:
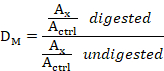
Where: DM is methylation dosage ratio, Ax is the area under peak x (e.g., L1-2m peak), and Actrl is the sum peak area of all seven control probes.
- Label the three methylation site peaks based on their approximate sizes (L1-1m at 153 bp, L1-2m at 119 bp, L1-3m at 133 bp). The remaining seven peaks correspond to the control probes.
Results
The main goal of this study was to evaluate the epigenetic effects of OS-EVs on MSCs. OS-EVs were isolated from HOS-143B cells using the standard differential centrifugation method. Expression of the typical EV markers CD63, Hsp70, and TSG101 by western blotting confirmed the presence of OS-EVs. (Figure 2A). Absence of calnexin signal indicated purity of the OS-EV isolate. Additional indication of purity was observed with TEM, with intact vesicles of various sizes being pres...
Discussion
This study illustrates how MSPA can be used to detect and quantify the methylation status of a specific genetic element. LINE-1 was the focus here, but the probes can be designed to target a range of genes and sequences. Moreover, there is a growing list of probe mixes available for different applications. MSPA is a simple and robust technique for DNA methylation analysis that does not require bisulfite conversion10. The complete procedure from sample preparation to data analysis takes around 2 da...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by University of Helsinki project funding (WBS490302, WBS73714112) Helsinki University Hospital State funding for university-level health research (Y1014SUL05, TYH2016130), Finnish-Norwegian Medical Foundation, and the Selma and Maja-Lisa Selander Fund (Minerva Foundation). We thank Walter Pavicic for providing the modified MSPA protocol and for related technical support. We are grateful to Teemu Masalin (University of Helsinki) for helping us with video production.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL syringe | Terumo | SS+01T1 | for NTA |
| 24-well plate | Corning | 3524 | MSC cell culture |
| 3730xl DNA Analyzer | Applied Biosystems, ThermoFisher Scientific | 3730XL | |
| 50 mL centrifuge tube | Corning | 430829 | |
| Beckman Optima LE-80K Ultracentrifuge | Beckman | ||
| BlueStar Prestained Protein Marker | Nippon Genetics | MWP03 | WB: protein marker |
| Calnexin (clone C5C9) | Cell Signaling Technology | 2679 | WB, dilution 1:800 |
| CD63 (clone H5C6) | BD Biosciences | 556019 | WB, dilution 1:1000 |
| Centrifuge 5702 R | Eppendorf | 5703000010 | For conditioned media and cells |
| Centrifuge 5810 | Eppendorf | 5810000010 | For spinning down 96-well plate |
| Centrifuge tube (polyallomer, 14x95 mm) | Beckman | 331374 | Ultracentrifugation |
| DMEM/F-12 + GlutaMAX medium | Gibco, Life Technologies | 31331-028 | For AT-MSC culture |
| Fetal bovine serum | Gibco, Life Technologies | 10270-106 | |
| GeneScan 500 LIZ size standard | Applied Biosystems, Life Technologies | 4322682 | for capillary electrophoresis |
| GenomePlex Complete Whole Genome Amplification (WGA) Kit | Sigma | WGA2-10RXN | for MSPA negative control |
| Hi-Di formamide | Applied Biosystems, Life Technologies | 4311320 | for capillary electrophoresis |
| HOS-143B cell line | ATCC | CRL-8303 | |
| Hsp70 (clone 5G10) | BD Biosciences | 554243 | WB, dilution 1:1000 |
| IRDye 800CW Goat anti-mouse | Li-Cor | 926-32210 | WB: secondary |
| IRDye 800CW Goat anti-rabbit | Li-Cor | 926-32211 | WB: secondary |
| LINE-1 probe-mix primers | IDT | Sequences in Table 1 | |
| MicroAmp Optical 96-well reaction plate with barcode | Applied Biosystems, Life Technologies | 4306737 | also requires sealing film |
| Micro BCA Protein Assay kit | ThermoFisher Scientific | 23235 | measure protein concentration |
| MiniProtean TGX 10% gels | Bio-Rad | 456-1034 | WB: gel electrophoresis |
| NanoSight LM14C | Malvern Instruments | for NTA | |
| Nitrocellulose membrane 0.2 µm | Bio-Rad | 1620112 | WB: protein transfer |
| NucleoSpin Tissue XS | Macherey-Nagel | 740901.50 | for DNA extraction |
| Odyssey Blocking Buffer | Li-Cor | 927-40000 | WB: blocking, antibodies |
| PBS, 1X | Corning | 21-040-CVR | |
| Penicillin-streptomycin | Gibco, Life Technologies | DE17-602E | Antibiotics for culture media |
| Protein LoBind tube, 0.5 mL | Eppendorf | 22431064 | For storing Evs |
| REVERT Total Protein Stain and Wash Solution Kit | Li-Cor | 926-11015 | WB: total protein staining |
| RKO cell line | ATCC | CRL-2577 | for MSPA positive control |
| RPMI medium 1640 + GlutaMAX | Gibco, Life Technologies | 61870-010 | For HOS-143B cell culture |
| SALSA MLPA HhaI enzyme | MRC-Holland | SMR50 | |
| SALSA MLPA reagent kit | MRC-Holland | EK1-FAM | |
| SALSA MLPA P300 probe-mix | MRC-Holland | P300-100R | |
| Swinging rotor SW-28 | Beckman Coulter | 342207 | Ultracentrifugation |
| Syringe filter, 0.22 µm | Jet Biofil | FPE-204-030 | sterile filtering FBS |
| Tecnai 12 | FEI Company | equipped with Gatan Orius SC 1000B CCD-camera (Gatan Inc., USA); for TEM | |
| TBS, 1X tablets | Medicago | 09-7500-100 | WB: buffer |
| Trans-Blot Turbo | Bio-Rad | WB: transfer | |
| Thermal cycler | ThermoFisher Scientific | TCA0096 | |
| TrypLE Express | Gibco | 12604-021 | for trypsinization of cells |
| TSG101 (clone 4A10) | Sigma | SAB2702167 | WB, dilution 1:500 |
References
- Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature Reviews Genetics. 8, 286-298 (2007).
- Herman, J. G., Baylin, S. B. Gene silencing in cancer in association with promoter hypermethylation. New England Journal of Medicine. 349, 2042-2054 (2003).
- Hanahan, D., Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell. 144 (5), 646-674 (2011).
- Howard, G., Eiges, R., Gaudet, F., Jaenisch, R., Eden, A. Activation and transposition of endogenous retroviral elements in hypomethylation induced tumors in mice. Oncogene. 27, 404-408 (2008).
- Lander, E. S., et al. Initial sequencing and analysis of the human genome. Nature. 409, 860-921 (2001).
- Rodriguez, J., et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Research. 66, 8462 (2006).
- Feinberg, A. P., Ohlsson, R., Henikoff, S. The epigenetic progenitor origin of human cancer. Nature Reviews Genetics. 7, 21-33 (2006).
- Bock, C., et al. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 21 (11), 4067-4068 (2005).
- Schouten, J. P., et al. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Research. 30, 57 (2013).
- Nygren, A. O. H., et al. Methylation-Specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Research. 33 (14), 128 (2005).
- El Andaloussi, S., Mager, I., Breakefield, X. O., Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery. 12, 347-357 (2013).
- Vallabhaneni, K. C., et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 6 (7), 4953-4967 (2015).
- Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A., Ratajczak, M. Z. Membrane- derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 20, 1487-1495 (2006).
- Lee, T. H., et al. Microvesicles as mediators of intercellular communication in cancer- the emerging science of cellular "debris". Seminars in Immunopathology. 33, 455-467 (2011).
- Kawamura, Y., Calle, A. S., Yamamoto, Y., Sato, T., Ochiya, T. Extracellular vesicles mediate the horizontal transfer of an active LINE-1 retrotransposon. Journal of Extracellular Vesicles. 8, 1643214 (2019).
- Mannerström, B., et al. Epigenetic alterations in mesenchymal stem cells by osteosarcoma-derived extracellular vesicles. Epigenetics. 14 (4), 352-364 (2019).
- Shelke, G. V., Lässer, C., Gho, Y. S., Lötvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. Journal of Extracellular Vesicles. 3, 24783 (2014).
- Wei, Z., Batagov, A. O., Carter, D. R., Krichevsky, A. M. Fetal bovine serum RNA interferes with the cell culture derived extracellular RNA. Scientific Reports. 6, 31175 (2016).
- Kornilov, R., et al. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. Journal of Extracellular Vesicles. 7, 1422674 (2018).
- Théry, C., Amigorena, S., Raposo, G., Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology. 30 (1), 1-29 (2006).
- Puhka, M., et al. Metabolomic profiling of extracellular vesicles and alternative normalization methods reveal enriched metabolites and strategies to study prostate cancer-related changes. Theranostics. 7 (16), 3824 (2017).
- Peltoniemi, H. H., et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: A prospective comparative study. Journal of Plastic, Reconstructive & Aesthetic Surgery. 66 (11), 1494-1503 (2013).
- Pavicic, W., Joensuu, E. I., Nieminen, T., Peltomäki, P. LINE-1 hypomethylation in familial and sporadic cancer. Journal of Molecular Medicine. 90, 827-835 (2012).
- . L1.2 sequence on GenBank (accession number: AH005269.2) Available from: https://www.ncbi.nlm.nih.gov/nuccore/AH005269 (2000)
- Designing synthetic MLPA probes (v04). MRC-Holland Available from: https://support.mlpa.com/downloads/files/designing-synthetic-mlpa-probes (2018)
- . Takara Bio Inc Available from: https://www.takarabio.com/us/products/cell_biology_and_epigenetics/epigenetics/dna_preparation/msre_overview (2018)
- Pisanic, R., et al. Long interspersed nuclear element 1 retrotransposons become deregulated during the development of ovarian cancer precursor lesions. The American Journal of Pathology. 189 (3), 513-520 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved