A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Doxorubicin-Induced Murine Model of Dilated Cardiomyopathy In Vivo
In This Article
Erratum Notice
Summary
Described is a protocol to establish a Doxorubicin-induced dilated cardiomyopathy (DCM) model in mice via long-term intraperitoneal injection of Doxorubicin.
Abstract
Dilated cardiomyopathy (DCM) refers to a spectrum of heterogeneous myocardial disorders characterized by ventricular dilation and depressed cardiac performance in the absence of hypertension, valvular, congenital, or ischemic heart diseases, and which may be related to infection, autoimmune or metabolic abnormalities, or family inheritance. It can progress into congestive heart failure with a poor prognosis. Doxorubicin (Dox) is widely employed as a chemotherapeutic drug, but its use is limited because it causes DCM-like changes of the myocardium. Its myocardial toxicity is attributed to oxidative stress, chronic inflammation, and cardiomyocyte apoptosis. A model of DCM exploiting these Dox-induced DCM symptoms has not been established.
Introduction
One of the most common causes of heart failure, DCM is characterized by ventricular dilatation and decreased cardiac function and is the most common reason for heart transplantation worldwide1. In order to further investigate its pathogenesis and find effective treatments, access to mature animal models is especially important. The purpose of the described experiments is to establish a stable mouse model of DCM that resembles human DCM.
Due to the complex pathogenesis of DCM, there are many different methods to make corresponding animal models. Spontaneous DCM models2 are relatively stable, but they are expensive and not easily available. Genetically modified animal models3 are not well-established and require more experimental use. DCM animal models induced by viral infection4 or autoimmune defects5 are easy to obtain, but they are not wholly representative of DCM. Models associated with myocardial toxicity include alcohol-induced DCM models and Dox-induced DCM animal models.
The Dox-induced cardiomyopathy model is obtained by intraperitoneal injection of Dox6. The model exploits the most severe chronic side effect of Dox: After Dox exposure, patients develop late onset DCM symptoms with clinical uniformity7. Dox-induced oxidative stress8 and mitochondrial damage9, which lead to cardiomyocyte apoptosis, are symptoms in the pathogenesis of DCM. There are acute and chronic Dox treatment models: a single high dose of Dox (15 mg/kg) induces a short-term model for cardiomyopathy10, while repetitive low-dose Dox injections (six weekly, 3 mg/kg) induce a long-term model for cardiomyopathy11. Based on the presented study, wild type mice intraperitoneally injected once a week for a month at a dose of 5 mg/kg display morphology and histology of the heart consistent with the characteristics of DCM by the end of the treatment, providing an ideal way to establish a DCM model.
Protocol
Animal experiments were approved by Institutional Animal Care and Use Committee (IACUC) of Nanjing Drum Tower Hospital.
1. Preparation of the reagents and animals
- Dissolve doxorubicin hydrochloride (Pfizer, USA) in sterilized water. Vortex to obtain a Dox solution of 1 mg/mL and keep at 4 °C.
- Use C57BL/6 mice (8–10 weeks old; 25–30 g weight). For this study, mice were purchased from the Model Animal Research Center of Nanjing University and kept in the animal room of Nanjing Drum Tower Hospital.
- Pathogen-free mouse cages were maintained under a 12 h light/dark cycle at a constant temperature of 23 °C. All animals were fed on a normal chow diet and got food and water ad libitum.
2. Establishment of DCM animal model
- Randomize mice into a normal (n = 5) group and Dox (n = 5) group.
- Administer the Dox solution intraperitoneally at a dose of 5 mg/kg using a 1 mL sterilized syringe 1x a week for the Dox group. Treat the control mice in the same way with the same amount of saline solution.
- Measure the body weight of the two groups weekly. Accordingly, adjust the injection dose based on body weight weekly for a total of 4 weeks, with a cumulative dose of 20 mg/kg (Figure 1).
NOTE: A time period of 4 weeks was chosen because echocardiography at 4 weeks showed a significant difference in cardiac function between the two groups.
3. Echocardiographic examination
- At the end of the fourth week, conduct an echocardiographic examination of the mice.
- Anesthetize the mice with 2% isoflurane intranasally. If the mice do not respond to a pinch of the skin with a toothed tweezer or stimulating the toes and tails, continue with the protocol.
- Place the mouse on an animal-handling platform in the supine position. To maintain the anesthesia, cover the animal's nose and mouth with a nose cone and deliver 2% isoflurane.
NOTE: For IP anesthesia, inject 4% chloral hydrate at a dose of 0.2 mL/20 g. - Remove chest fur carefully with an electric shaver. Assess cardiac function in vivo using transthoracic echocardiography.
- Perform the LV echocardiogram in both the parasternal long-axis and short-axis views at a frame rate of 233 Hz. End-systolic and end-diastolic dimensions were defined as the phases corresponding to the ECG T wave and to the R wave, respectively.
- On the M-mode tracings, measure the average LV end-systolic diameter (LVIDd), LV end-diastolic diameter (LVIDs), interventricular septal thickness (IVS), and LV posterior wall thickness (LVPW) from 3–5 heart beats. Also calculate the ejection fraction (EF) and fraction shortening (FS) based on echocardiography.
4. Histological staining
- After the echocardiographic analysis, sacrifice the mice by intraventricular injection of 10% potassium chloride.
- Perfuse the heart with about 30 mL of saline after dissection until the liver and lung become pale.
- Excise the heart and thoroughly wash it in phosphate buffer solution to extrude blood.
- Fix the heart in 4% formalin at room temperature for 24 h and treat the tissue in a paraffin box so that the paraffin wax cools down and solidifies.
- Cut the hearts into 5 μm thick slices for pathological staining.
- Dewax and rehydrate sections containing papillary muscle.
- Incubate slides at 55 °C for 30 min. Then, incubate in xylene 2x for 2 min each; 100% ethanol 2x for 2 min each; 95% ethanol 2x for 2 min each; 80% ethanol for 2 min; 75% ethanol for 2 min; and 50% ethanol for 2 min.
- Stain using hematoxylin and eosin (H&E) as well as Masson’s stain.
Results
Cardiac function
Dilated cardiomyopathy is characterized by progressive ventricular dilatation and contractile dysfunction. Figure 2 shows representative echocardiographic images of the two groups. Dox-treated mice showed markedly reduced left ventricular ejection fraction and left ventricular fractional shortening (Figure 3A,B). The LV diameter also increased in both the diastolic and systolic phases (
Discussion
Dox is a nonspecific periodic antitumor chemotherapy drug commonly used in clinical practice12. Its main side effect is cardiotoxicity, characterized by cardiomyopathy and subsequent heart failure13. The underlying mechanism includes damage of myocardial lipid peroxidation, inhibition of myocardial sarcoplasmic reticulum Ca2+-ATPase activity, and activation of the myocardial local renin-angiotensin system, resulting in increased AT II production and intracellular...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by Key Project Medical Science and Technology Development Foundation, Nanjing Department of Health (No.YKK16098).
Materials
| Name | Company | Catalog Number | Comments |
| 4% paraformaldehyde | servicebio | CAS30525-89-4 | |
| C57BL/6 mice | Model Animal Research Center of Nanjing University | \ | |
| Doxorubicin hydrochloride | Pfizer | CAS25316-40-9 | |
| echocardiography | Visualsonics | \ | |
| Hematoxylin and Eosin staining kit | Solarbio | G1120 | |
| Masson staining kit | Solarbio | G1343 | |
| phosphate buffer solution | Sigma | P5368 | |
| potassium chloride | Sigma | CAS7447-40-7 | |
| sterilized syringe | Millipore | SLGP033RB |
References
- Weintraub, R. G., Semsarian, C., MacDonald, P. Dilated cardiomyopathy. Lancet. 390 (10092), 400-414 (2017).
- Ichihara, S., et al. Attenuation of oxidative stress and cardiac dysfunction by bisoprolol in an animal model of dilated cardiomyopathy. Biochemical and Biophysical Research Communications. 350 (1), 105-113 (2006).
- Fountoulakis, M., et al. Alterations in the heart mitochondrial proteome in a desmin null heart failure model. Journal of Molecular and Cellular Cardiology. 38 (3), 461-474 (2005).
- Fairweather, D., Rose, N. R. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 41 (1), 118-122 (2007).
- Wang, Z. H., et al. A therapeutic anti-CD4 monoclonal antibody inhibits T cell receptor signal transduction in mouse autoimmune cardiomyopathy. Chinese Medical Journal. 120 (15), 1319-1325 (2007).
- Riad, A., et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. European Journal of Heart Failure. 10 (3), 233-243 (2008).
- Kankeu, C., Clarke, K., Passante, E., Huber, H. J. Doxorubicin-induced chronic dilated cardiomyopathy-the apoptosis hypothesis revisited. Journal of Molecular Medicine. 95 (3), 239-248 (2017).
- Zhao, L., et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biology. 15, 284-296 (2018).
- O'Connell, J. L., et al. Short-term and long-term models of doxorubicin-induced cardiomyopathy in rats: A comparison of functional and histopathological changes. Experimental and Toxicologic Pathology. 69 (4), 213-219 (2017).
- Yuan, Y. P., et al. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. Journal of Molecular and Cellular Cardiology. 114, 38-47 (2018).
- Sun, Z., et al. The TGF-beta pathway mediates doxorubicin effects on cardiac endothelial cells. Journal of Molecular and Cellular Cardiology. 90, 129-138 (2016).
- Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., Gianni, L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 56 (2), 185-229 (2004).
- Vejpongsa, P., Yeh, E. T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. Journal of The American College of Cardiology. 64 (9), 938-945 (2014).
- Renu, K., V, G. A., P, B. T., Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy - An update. European Journal of Pharmacology. 818, 241-253 (2018).
Erratum
Formal Correction: Erratum: A Doxorubicin-Induced Murine Model of Dilated Cardiomyopathy In Vivo
Posted by JoVE Editors on 11/04/2021. Citeable Link.
An erratum was issued for: A Doxorubicin-Induced Murine Model of Dilated Cardiomyopathy In Vivo. A figure was updated.
Figure 1 was updated from:
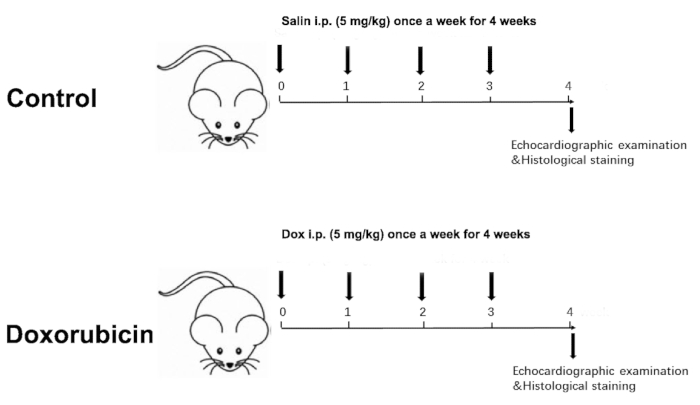
Figure 1: Schematic diagram of a Dox-induced dilated cardiomyopathy. Please click here to view a larger version of this figure.
to:
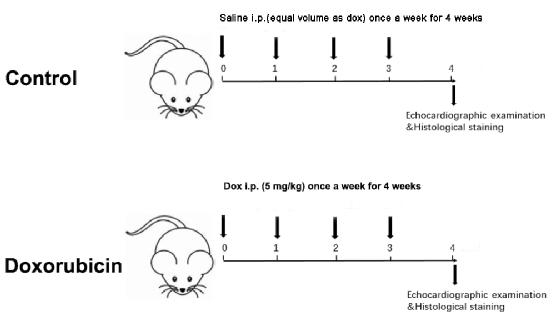
Figure 1: Schematic diagram of a Dox-induced dilated cardiomyopathy. Please click here to view a larger version of this figure.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved