A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Induction of Diffuse Axonal Brain Injury in Rats Based on Rotational Acceleration
* These authors contributed equally
In This Article
Summary
This protocol validates a reliable, easy-to-perform and reproducible rodent model of brain diffuse axonal injury (DAI) that induces widespread white matter damage without skull fractures or contusions.
Abstract
Traumatic brain injury (TBI) is a major cause of death and disability. Diffuse axonal injury (DAI) is the predominant mechanism of injury in a large percentage of TBI patients requiring hospitalization. DAI involves widespread axonal damage from shaking, rotation or blast injury, leading to rapid axonal stretch injury and secondary axonal changes that are associated with a long-lasting impact on functional recovery. Historically, experimental models of DAI without focal injury have been difficult to design. Here we validate a simple, reproducible and reliable rodent model of DAI that causes widespread white matter damage without skull fractures or contusions.
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability in the United States. TBIs contribute to about 30% of all injury-related deaths1,2. The leading causes of TBI differ among age groups and include falls, high-speed collisions during sports, intentional self-harm, motor vehicle crashes and assaults1,2,3.
Brain diffuse axonal injury (DAI) is a specific type of TBI induced by rotational acceleration, shaking or blast injury of the brain resulting from unrestricted head movement in the instant after injury4,5,6,7,8. DAI involves widespread axonal damage leading to long-lasting neurological impairment that is associated with poor outcome, burdensome health-care costs, and a 33-64% mortality rate1,2,4,5,9,10,11. Despite significant recent research into the pathogenesis of DAI, there has not been a consensus on best treatment options11,12,13,14.
Over the last decades, numerous experimental models have attempted to accurately replicate different aspects of DAI11,12,15,16. However, these models have limitations given the unique presentation of DAI compared to other focal injuries. These prior models not only cause axonal injury in white matter regions but also result in focal cerebral injuries. Clinically, DAI is accompanied by micro hemorrhages, which may constitute a major cause of damage to white matter.
Only two animal models have been shown to replicate the key clinical features of DAI. Gennarelli and colleagues produced the first lateral head rotation device in 1982, using nonimpact head rotational acceleration to induce coma with DAI in a nonhuman primate model15. This primate model employed controlled single rotation for acceleration and deceleration to displace the head through 60° within 10-20 ms. This technique was able to emulate impaired consciousness and widespread axonal damage that resembled the effects of severe TBI observed in human brains. However, primate models are very expensive4,11,16. Based in part on the previous model, a pig model of rotational acceleration brain injury was designed in 1994 (Ross et al.) with similar results14.
These two animal models, though they produced different presentations of typical pathology, have added greatly to the concepts of DAI pathogenesis. Rapid head rotation is generally accepted as the best method for inducing DAI, and rodents provide a less expensive model for the rapid head rotation studies11,16. Here, we validate a simple, reproducible and reliable rodent model of DAI that causes widespread white matter damage without skull fractures or contusions. This current model will enable better understanding of the pathophysiology of DAI and development of more effective treatments.
Protocol
The experiments were performed following the recommendations of the Declarations of Helsinki and Tokyo and to the Guidelines for the Use of Experimental Animals of the European Community. The experiments were approved by the Animal Care Committee of Ben-Gurion University of the Negev.
1. Preparing rats for the experimental procedure
NOTE: Select adult male Sprague-Dawley rats weighing 300-350 g.
- Obtain approval for performing these experiments from the Institutional Animal Care and Use Committee.
- Maintain rats at a room temperature of 22 ± 1 °C, with 12 hour light and 12 hour dark cycles. Provide rat chow and water ad libitum.
- Perform all experiments between 6:00 a.m. and 12:00 p.m.
- Use a continuous isoflurane administration system to induce anesthesia. Make sure the vaporizer system is filled with isoflurane.
- Anesthetize the rats with 2% isoflurane.
- Confirm that the rat is fully anesthetized by observing a lack of movement or pedal reflex in response to an external stimuli.
2. Induction of diffuse axonal injury
NOTE: The device consists of the following components: 1) transparent plastic cylinder, 2) iron weight (1308 g), 3) rotation mechanism consisting of a cylindrical tube, two bearings upon which the axis rotate and a head fixation (for ear pins); 4) horizontal platform on which are fixed two bearings.
- Place the device on a heavy, stable laboratory table.
- Attach the weight to a string that is elevated to a height of 120 cm.
- Allow the freely falling weight to hit the bolt, activating the rotational mechanism. Using the lateral head rotation device, the rodent’s head is turned rapidly from 0 to 90°.
- After induction of diffuse axonal brain injury, transfer the rat to a recovery room.
3. Measurement of rotational Kinematics/Biomechanical parameters.
- Measure rotational kinematics/biomechanical parameters as follows:
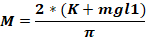
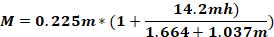

where Fo - force applied to animal head (kg); M – moment of force; K – kinetic energy; m – mass of the falling weight; g - gravitational acceleration; h – height (cm); D – distance between the ear pins (cm).
NOTE: To calculate the force applied to the animal’s head (Fo), it is necessary to know the mass of the falling weight, the height at which the weight falls, and the distance between the ear pins. The other parameters remain unchanged.
4. Evaluation of Neurological Severity Score after 48 hours
NOTE: Neurological deficits were assessed and graded using a Neurological Severity Score, as previously described17,18,19. Alterations in motor function and behavior are assessed by a point-system such that a maximum score of 24 represents severe neurological dysfunction. A score of 0 indicates intact neurological status. The following behavioral functions are assessed.
- Assess the rat’s inability to exit from a circle (50 cm in diameter) when left in its center. Perform this for three individual sessions lasting 30 min, 60 min, and more than 60 min.
- Test the rat for a loss of righting reflex in three sessions lasting 20 min, 40 min, and over 60 min.
- Perform the test for hemiplegia, the inability of the rat to resist forced changes in position.
- Raise the rat by its tail to test the flexion of the hindlimb.
- Place the rat on the floor to test its ability to walk straight.
- Test for three separate reflexes: the pinna reflex, the corneal reflex, and the startle reflex.
- Rate the rat with a clinical grade based on loss of seeking behavior and prostration.
- Test limb reflexes for placement. Perform the test on the left and right forelimbs, and then the left and right hindlimbs.
- Perform a functional test via the beam balancing task. Beam should measure 1.5 cm wide. Run the test for sessions of 20 s, 40 s, and more than 60 s.
- Perform beam walking test on the rat with beams of three different widths: 8.5 cm wide, 5 cm wide, and 2.5 cm wide.
5. Brain collection for histological examination after 48 hours
- At 48 hours post injury, euthanize the rats by replacing their inspired gas mixture with 20% O2/80% CO2. Ensure that CO2 is delivered at a predetermined rate in accordance with Institutional Animal Care and Use Committee guidelines.
- Ensure death confirmation in accordance with Institutional Animal Care and Use Committee guidelines.
- Transcardiacally perfuse the rat with 0.9% heparinized saline at temperature 4 °C, followed by 500 mL of 4% paraformaldehyde in 0.1 M phosphate buffer saline (pH 7.4).
- After perfusion, perform decapitation with a guillotine.
- Perform brain collection by removing the calvarias with bone-cutting forceps to avoid damaging brain tissue.
- Remove the brain immediately and fix in a 4% buffered formaldehyde solution for 48 h at 4 °C.
- Block brains into 5 mm coronal sections from the olfactory bulb face to the visual cortex and bisect cerebellums and brain stems.
- Following paraffin embedding, cut coronal and sagittal sections (5 µm) away from the thalamus by microtome sectioning.
6. Immunochemical staining and examination
- Gently place the slices on glass slides with a soft brush, 1 slice per slide.
- Produce immunochemical staining of βAPP.
- Deparaffinize slices with xylene (3 times for 5 min each) and rehydrate with gradually-reduced concentrations of ethanol at room temperature: 3 min in 100% ethanol twice, 3 min in 95% ethanol twice, 3 min in 90% ethanol, 3 min in 70% ethanol, and 3 min in DDW.
- Treat deparaffinized and rehydrated brain sections with 3% H2O2 for 15 min at room temperature to block endogenous peroxidase activity.
- Incubate sections with 0.01 M sodium citrate (pH 6.0) at 98 °C for 5 min for antigen retrieval.
- Keep the slides in the buffer for 20 min at room temperature to cool.
- Wash sections with phosphate-buffered saline (PBS) solution twice for 5 min.
- Block the sections with 2.5% normal horse serum for 1 h at room temperature and incubate overnight at 4 °C in primary rabbit anti-APP (1:4000) diluted in the blocking serum.
- After incubation in primary antibody, wash sections in PBS at room temperature.
- Incubate sections in appropriately diluted biotinylated secondary antibody for 15 min and wash with PBS for 3 min twice at room temperature.
- Incubate in streptavidin–peroxidase for 15 min and wash again in PBS for 3 min twice at room temperature.
- Incubate sections with buffered substrate solution (pH 7.5) containing hydrogen peroxide and 3,3-diaminobenzidine chromogen solution and protect from light until the color is developed.
- Incubate the slides with DDW at room temperature for 5 min in order to stop the reaction.
- Counterstain sections with Hematoxylin for 3 min at room temperature and wash for 5 min with flowing tap water.
- Dehydrate the slides with gradually increasing concentrations of ethanol at room temperature: 2 min in DDW, 2 min in 70% ethanol, 2 min in 90% ethanol, 2 min in ethanol 95%, 2 min in 100% ethanol, and 3 min in xylene three times.
- Dry and mount with mounting medium.
- Examine the slices under microscope magnification of 200x with a 20 mm objective lens using a microscope.
Results
Table 1 illustrates the protocol timeline. The mortality rate in this model of DAI was 0%. A Mann-Whitney test indicated that neurological deficit was significantly greater for the 15 DAI rats compared to the 15 sham rats at 48 hours following intervention (Mdn = 1 vs. 0), U = 22.5, p < 0.001, r = 0.78 (see Table 2). The data are measured in counts and are presented as median and 25–75 percentile range.
Representative photomicrographs of thalamic sec...
Discussion
This protocol describes a rodent model of DAI. In DAI, rotational acceleration on the brain causes a shear effect that triggers axonal and biochemical changes that lead to loss of axonal function in a progressive process. Secondary axonal changes are produced by a rapid axonal stretch injury and are variable in their extent and severity4,5,10. Within hours to days after the primary injury, biochemical changes will lead to the lo...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge Dr. Nathan Kleeorin (Department of Mechanical Engineering, Ben-Gurion University of the Negev) for his assistance with the biomechanical measurements. Also, we thank Professor Olena Severynovska, Maryna Kuscheriava, Maksym Kryvonosov, Daryna Yakumenko and Evgenia Goncharyk of the Department of Physiology, Faculty of Biology, Ecology, and Medicine, Oles Honchar Dnipro University, Dnipro, Ukraine for her support and helpful contributions to our discussions.
Materials
| Name | Company | Catalog Number | Comments |
| 0.01 M sodium citrate | SIGMA - ALDRICH | ||
| 2.5% normal horse serum | SIGMA - ALDRICH | H0146 | Liquid |
| 4 % buffered formaldehyde solution | |||
| Anti-Amyloid Precursor Protein, C - terminal antibodyproduced in rabbit | SIGMA - ALDRICH | Lot 056M4867V | |
| biotinylated secondary antibody | Vector | BA-1000-1.5 | 10 mM sodium phosphate, pH 7.8, 0.15 M NaCl, 0.08% sodium azide, 3 mg/ml bovine serum albumin |
| bone-cutting forceps | |||
| DAB Peroxidase (HRP) Substrate Kit (with Nickel), 3,3’-diaminobenzidine | vector laboratory | ||
| embedding cassettes | |||
| ethanol 99.9 % | ROMICAL | Flammable Liquid | |
| guillotine | |||
| Hematoxylin | SIGMA - ALDRICH | H3136-25G | |
| Hydrogen peroxide solution | Millipore | 88597-100ML-F | |
| Isofluran, USP 100% | Piramamal Critical Care, Inc | ||
| Olympus BX 40 microscope | Olympus | ||
| paraffine | paraplast plus leica biosystem | Tissue embedding medium | |
| phosphate-buffered saline (PBS) | SIGMA - ALDRICH | P5368-10PAK | Contents of one pouch, when dissolved in one liter of distilled or deionized water, will yield 0.01 M phosphate buffered saline (NaCl 0.138 M; KCl - 0.0027 M); pH 7.4, at 25 °C. |
| Streptavidin HRP | ABCAM | ab64269 | Streptavidin-HRP for use with biotinylated secondary antibodies during IHC / immunohistochemistry. |
| xylene |
References
- Faul, M., Wald, M. M., Xu, L., Coronado, V. G. Traumatic brain injury in the United States; emergency department visits, hospitalizations, and deaths, 2002-2006. US Government. , (2010).
- Taylor, C. A., Bell, J. M., Breiding, M. J., Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveillance Summaries. 66, 1-16 (2017).
- Peterson, A. B., Xu, L., Daugherty, J., Breiding, M. J. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths, United States, 2014. US Government. , (2014).
- Su, E., Bell, M. Diffuse axonal injury. Translational Research in Traumatic Brain Injury. 57, 41 (2016).
- Hammoud, D. A., Wasserman, B. A. Diffuse axonal injuries: pathophysiology and imaging. Neuroimaging Clinics. 12, 205-216 (2002).
- Adams, J. H., Graham, D. I., Gennarelli, T. A., Maxwell, W. L. Diffuse axonal injury in non-missile head injury. Journal of Neurology, Neurosurgery, and Psychiatry. 54, 481-483 (1991).
- Slazinski, T., Johnson, M. C. Severe diffuse axonal injury in adults and children. Journal of Neuroscience Nursing. 26, 151-154 (1994).
- Gentleman, S. M., et al. Axonal injury: a universal consequence of fatal closed head injury. Acta Neuropathologica. 89, 537-543 (1995).
- Marehbian, J., Muehlschlegel, S., Edlow, B. L., Hinson, H. E., Hwang, D. Y. Medical Management of the Severe Traumatic Brain Injury Patient. Neurocritical Care. 27, 430-446 (2017).
- Adams, J. H., et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 15, 49-59 (1989).
- Xiao-Sheng, H., Sheng-Yu, Y., Xiang, Z., Zhou, F., Jian-ning, Z. Diffuse axonal injury due to lateral head rotation in a rat model. Journal of Neurosurgery. 93, 626-633 (2000).
- Ross, D. T., Meaney, D. F., Sabol, M. K., Smith, D. H., Gennarelli, T. A. Distribution of forebrain diffuse axonal injury following inertial closed head injury in miniature swine. Experimental Neurology. 126, 291-299 (1994).
- Bullock, R. Opportunities for neuroprotective drugs in clinical management of head injury. Journal of Emergency Medicine. 11, 23-30 (1993).
- Gennarelli, T. A. Mechanisms of brain injury. Journal of Emergency Medicine. 11, 5-11 (1993).
- Gennarelli, T. A., et al. Diffuse axonal injury and traumatic coma in the primate. Annals of Neurology. 12, 564-574 (1982).
- Xiaoshengi, H., Guitao, Y., Xiang, Z., Zhou, F. A morphological study of diffuse axonal injury in a rat model by lateral head rotation trauma. Acta Neurologica Belgica. 110, 49-56 (2010).
- Zlotnik, A., et al. beta2 adrenergic-mediated reduction of blood glutamate levels and improved neurological outcome after traumatic brain injury in rats. Journal of Neurosurgical Anesthesiology. 24, 30-38 (2012).
- Boyko, M., et al. An Alternative Model of Laser-Induced Stroke in the Motor Cortex of Rats. Biological Procedures Online. 21, 9 (2019).
- Boyko, M., et al. The neuro-behavioral profile in rats after subarachnoid hemorrhage. Brain Research. 1491, 109-116 (2013).
- Ma, J., Zhang, K., Wang, Z., Chen, G. Progress of Research on Diffuse Axonal Injury after Traumatic Brain Injury. Neural Plasticity. 2016, 9746313 (2016).
- Medana, I. M., Esiri, M. M. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 126, 515-530 (2003).
- Tang-Schomer, M. D., Johnson, V. E., Baas, P. W., Stewart, W., Smith, D. H. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Experimental Neurology. 233, 364-372 (2012).
- Johnson, V. E., Stewart, W., Smith, D. H. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease. Nature Reviews Neuroscience. 11, 361-370 (2010).
- Sherriff, F. E., Bridges, L. R., Sivaloganathan, S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathologica. 87, 55-62 (1994).
- Reichard, R. R., White, C. L., Hladik, C. L., Dolinak, D. Beta-amyloid precursor protein staining of nonaccidental central nervous system injury in pediatric autopsies. Journal of Neurotrauma. 20, 347-355 (2003).
- Gentleman, S. M., Nash, M. J., Sweeting, C. J., Graham, D. I., Roberts, G. W. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neuroscience Letters. 160, 139-144 (1993).
- Smith, D. H., Hicks, R., Povlishock, J. T. Therapy development for diffuse axonal injury. Journal of Neurotrauma. 30, 307-323 (2013).
- McKenzie, K. J., et al. Is beta-APP a marker of axonal damage in short-surviving head injury. Acta Neuropathologica. 92, 608-613 (1996).
- Wilkinson, A., Bridges, L., Sivaloganathan, S. Correlation of survival time with size of axonal swellings in diffuse axonal injury. Acta Neuropathologicaogica. 98, 197-202 (1999).
- Thompson, H. J., et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. Journal of Neurotrauma. 22, 42-75 (2005).
- Alder, J., Fujioka, W., Lifshitz, J., Crockett, D. P., Thakker-Varia, S. Lateral fluid percussion: model of traumatic brain injury in mice. Journal of Visualized Experiments. , e3063 (2011).
- Povlishock, J., Marmarou, A., McIntosh, T., Trojanowski, J., Moroi, J. Impact acceleration injury in the rat: evidence for focal axolemmal change and related neurofilament sidearm alteration. Journal of Neuropathology & Experimental Neurology. 56, 347-359 (1997).
- Heath, D. L., Vink, R. Impact acceleration-induced severe diffuse axonal injury in rats: characterization of phosphate metabolism and neurologic outcome. Journal of Neurotrauma. 12, 1027-1034 (1995).
- Lighthall, J. W. Controlled cortical impact: a new experimental brain injury model. Journal of Neurotrauma. 5, 1-15 (1988).
- Palmer, A. M., et al. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. Journal of Neurochemistry. 61, 2015-2024 (1993).
- Hamm, R. J., et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. Journal of Neurotrauma. 9, 11-20 (1992).
- Nyanzu, M., et al. Improving on Laboratory Traumatic Brain Injury Models to Achieve Better Results. International Journal of Medical Sciences. 14, 494-505 (2017).
- Xiong, Y., Mahmood, A., Chopp, M. Animal models of traumatic brain injury. Nature Reviews Neuroscience. 14, 128-142 (2013).
- Lighthall, J. W., Dixon, C. E., Anderson, T. E. Experimental models of brain injury. Journal of Neurotrauma. 6, 83-97 (1989).
- Meaney, D. F., et al. Modification of the cortical impact model to produce axonal injury in the rat cerebral cortex. Journal of Neurotrauma. 11, 599-612 (1994).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved