A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Analysis and Specification of Starch Granule Size Distributions
In This Article
Summary
Presented here is a procedure for reproducible and statistically valid determinations of starch granule size distributions, and for specifying the determined granule lognormal size distributions using a two-parameter multiplicative form. It is applicable to all granule sizing analyses of gram-scale starch samples for plant and food science research.
Abstract
Starch from all plant sources are made up of granules in a range of sizes and shapes having different occurrence frequencies, i.e., exhibiting a size and a shape distribution. Starch granule size data determined using several types of particle sizing techniques are often problematic due to poor reproducibility or lack of statistical significance resulting from some insurmountable systematic errors, including sensitivity to granule shapes and limits of granule-sample sizes. We outlined a procedure for reproducible and statistically valid determinations of starch granule size distributions using the electrical sensing zone technique, and for specifying the determined granule lognormal size distributions using an adopted two-parameter multiplicative form with improved accuracy and comparability. It is applicable to all granule sizing analyses of gram-scale starch samples, and, therefore, could facilitate studies on how starch granule sizes are molded by the starch biosynthesis apparatus and mechanisms; and how they impact properties and functionality of starches for food and industrial uses. Representative results are presented from replicate analyses of granule size distributions of sweetpotato starch samples using the outlined procedure. We further discussed several key technical aspects of the procedure, especially, the multiplicative specification of granule lognormal size distributions and some technical means for overcoming frequent aperture blockage by granule aggregates.
Introduction
Starch granules are the physical structure in which two main reserve homoglucan polymers in plant photosynthesis and storage tissues, the linear or sparsely branched amylose and the highly branched amylopectin, are orderly packed along with some minor components, including lipids and proteins. Starch granules from various plant species exhibit many three-dimensional (3D) shapes (reviewed in ref.1,2), including spheres, ellipsoids, polyhedrons, platelets, cubes, cuboids, and irregular tubules. Even those from the same tissue or different tissues of the same plant species could have a set of shapes with varying occurrence frequencies. In other words, starch granules from a plant species may have a characteristic statistical shape distribution, rather than a specific shape. The non-uniform and non-spherical granule shapes make it difficult to properly measure and define starch granule sizes. Additionally, starch granules from the same tissues of a plant species are of a range of sizes with different proportions, i.e., exhibiting a characteristic size distribution. This size distribution further complicates the analysis and description of starch granule sizes.
Starch granule sizes have been analyzed using several categories of particle sizing techniques (reviewed in ref.3), including microscopy, sedimentation/steric field-flow fractionation (Sd/StFFF), laser diffraction and electrical sensing zone (ESZ). However, these techniques are not equally suited for the determination of starch granule sizes in the presence of a granule shape and a size distribution. Microscopy, including light, confocal and scanning electron microscopy, is excellent for the studies of morphology4,5,6,7, structure8,9 and development10,11 of starch granules, but hardly suited for defining their size distributions due to some inherent shortcomings. Direct measurements of microscopic granule images or software-assisted image analysis of optical microscopy data (IAOM), which have been used for the determination of granule sizes of starches from several species, including maize12, wheat13,14, potato15 and barley16, can measure only 1D (usually maximal length) or 2D (surface area) sizes of very limited numbers (tens to a few thousands) of starch granule images. The small granule sampling sizes that are inherently constrained by the techniques could rarely be statistically representative, considering the enormous granule numbers per unit weight of starch (~120 x 106 per gram, assuming all 10 µm spheres at 1.5 g/cm³ density), and, therefore, could lead to the poor reproducibility of the results. The Sd/StFFF technique may have high speed and resolution, and narrow size fractions of starch granules17, but has been rarely used probably because its accuracy could be severely affected by damages, different shapes, and density of starch granules. The laser diffraction technique is the most widely used, and has been applied for starch granule size analyses for all major crop species3,14,16. Although the technique has many advantages, it is actually not suited for determinations of starch granule sizes in the presence of a granule shape distribution. Most of the concurrent laser diffraction instruments rely on the Mie light-scattering theory18 for uniform spherical particles and the modified Mie theory18 for some other shapes of uniformity. The technique is, therefore, inherently very sensitive to particle shapes, and not entirely suited even for certain shapes of uniformity19, let alone for starch granules having a set of shapes of varying proportions. The ESZ technique measures the electric field disturbance proportional to the volume of the particle passing through an aperture. It provides granule volume sizes, as well as the number and volume distribution information, etc., at high resolutions. Since the ESZ technique is independent of any optical properties of particles including color, shape, composition or refractive index, and results are very reproducible, it is particularly suited for determining size distributions of starch granules having a set of shapes.
Starch granule sizes have also been defined by using many parameters. They were often simplistically described by average diameters, which in some cases were the arithmetic means of the microscopically measured maximal lengths of 2D images12,20, or averages of equivalent sphere diameters3. In other cases, the granule size distributions were specified by using size ranges21,22, the distribution mean volume or mean diameter (sphere equivalent, weighted by number, volume, or surface area) assuming a normal distribution14,23,24,25,26. These descriptors of starch granule sizes from various analyses are of a vastly different nature, and not strictly comparable. It could be very misleading if these “sizes” of starch granules from different species or even the same tissues of the same species were directly compared. Furthermore, the spread (or shape) parameter of the assumed normal distributions, i.e., the standard deviation σ (or graphic standard deviation σg) measuring the width of the distribution (i.e., the spread of the sizes), has been ignored in most studies.
To resolve the aforementioned critical issues facing starch granule sizing analyses, we outlined a procedure for reproducible and statistically valid determinations of granule size distributions of starch samples using the ESZ technique, and for properly specifying the determined granule lognormal size distributions using an adopted two-parameter multiplicative form27 with improved accuracy and comparability. For validation and demonstration, we performed replicate granule sizing analyses of sweetpotato starch samples using the procedure, and specified the lognormal differential volume-percentage volume-equivalent-sphere diameter distributions using their graphic geometric means 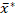 and multiplicative standard deviations s* in a
and multiplicative standard deviations s* in a 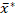 x/ (multiply and divide) s* form.
x/ (multiply and divide) s* form.
Protocol
1. Preparation of starch samples
- Prepare two (or three) gram-scale replicate starch samples from starch-accumulating tissues of various plant species following the established procedures (e.g., potatoes15, sweetpotatoes28, wheat grains13,29, and maize kernels30, etc.).
- Thoroughly wash starch samples with acetone or toluene 3-4x to minimize granule aggregates and dry them completely.
NOTE: Use extraction procedures that yield more than 1 g of starch per preparation. One or two 0.5-g aliquots from each of the three or two replicate extracts, respectively, are sampled for granule sizing analysis of one starch extract.
2. Electrolyte preparation
- Prepare 500 mL of 50 g/L lithium chloride in methanol for four sizing runs for replication starch samples (100 mL per run plus an extra 100 mL). Preferably, make the electrolyte in large-volume batches, e.g., 4 to 8 L at a time, to minimize the concentration variation.
- Cool the container on ice or in a 4 °C cabinet to speed up dissolution of the lithium chloride.
3. Setting up the analyzer
- Choose an aperture tube with a particle diameter range covering the known (in the literature or through trial runs) granule size range of starch samples to be analyzed, e.g., a 100 μm aperture for sweetpotato starches. For starch samples of unknown granule size range, select an appropriate aperture through trial runs using several aperture tubes having overlapping particle diameter ranges.
NOTE: The particle diameter range of an aperture tube is its accurate sizing range between 2 to 60% of, and with an extended sizing range to 80% of its orifice diameter. Table 1 lists properties of three most useful aperture tubes for sizing granules of major crop starches. If the granule size range of a starch sample is wider than the sizing range of a single aperture tube, perform a multi-tube overlap analysis combining up to five particle size distributions measured with apertures of different sizes. Each aperture is identifiable by its diameter and part number labeled on the tube. Its diameter and serial number contained in a barcode on the tube can be scanned into the analyzer software using the Bar Code Reader on the Control Panel of the analyzer. - Chose a 100 or 200 mL analytical beaker (over cuvettes) for the determination of starch granule sizes, and set up automatic stirring (below) to maintain a good granule suspension during measurement.
- Create a Standard Operating Method (SOM) to specify run settings, and a Preferences file for analyzing, viewing, and printing the results. Combine SOM and Preferences file into a Standard Operating Procedure (SOP) as needed.
NOTE: For non-standardizable analyses, use SOM to run the analyses, and adjust the SOM settings between runs through the Edit the SOM window (see below) as needed. After run completion, analyze, view, and print the run results by changing the Preferences as desired. For standardizable granule sizing analyses, use an SOP to run the analyses.- Start the analyzer software. On the Main Manu, click SOP | Create SOM Wizard or Edit the SOM, or on the Status Panel, click Edit SOM. Use the wizard or the Edit the SOM window to select settings for an SOM. Settings typically used for sizing granules of sweetpotato starch samples are summarized in Table 2.
- Save the created SOM to a File in the SOM Wizard-Summary of Settings window, or in the Edit the SOM window.
- On the Main Manu, Click SOP | Create Preferences Wizard or Edit Preferences. Use the wizard or tabs in the Preferences edit window to select preference settings as those in Table 3 or others as desired.
- Save the selected Preferences to a file in the Create Preferences Wizard-Summary of Settings window or in the Edit Preferences window.
- On the Main Menu, click SOP | Create SOP Wizard. Following the step-by-step guide of the wizard, enter a description, select the SOM and Preferences file to create and save an SOP.
4. Granule sizing analyses of the starch samples
- Prepare the Analyzer
- Turn on the analyzer, open the software in the computer and verify the Ready status at the top of the Status Panel after its automatic connection to the analyzer.
- Fill the electrolyte jar with electrolyte, empty the waste jar if necessary.
- Properly install and secure the chosen aperture tube following the guide in the user’s manual. For an uncalibrated new aperture tube, calibrate it following the step-by-step guide under Calibration | Calibrate Aperture on the Main Menu. For a calibrated aperture tube, verify the calibration following the step-by-step guide of the Change Aperture Tube Wizard under the Run or Calibration | Verify Aperture Calibration on the Main Menu.
- Unlock the assay platform by pushing the lock-release clip (on the middle front of the left sample compartment wall) and manually lower the platform to the bottom. Place an analytical beaker containing 100 mL of electrolyte on the platform, move the stirrer to the stirring position, and manually raise the platform to the self-locking upper position to immerse the aperture tube and stirrer in the electrolyte.
- Click Fill on the bottom instrument Toolbar to have the analyzer automatically fill the system with the electrolyte and click Flush to have the analyzer automatically flush the system.
- Load the SOM by clicking SOP | Load an SOM on the Main Menu, and use the SOM to run an analysis without a Preferences file. Alternatively, load an SOP by clicking SOP | Load an SOP on the Main Menu or Load SOP on the Status Panel, and use the SOP to run an analysis.
- If using an SOP, click SOP | SOM Info or Preference Info on the Main Menu to verify the SOM and Preference settings. Click Sample | Enter Sample Info on the Main Menu or Edit Info on the Status Panel to enter the sample information for the run.
- Prepare starch-methanol sample and sizing suspensions
- Weigh two or one 0.5 g sample from each of the two or three replicate starch extracts, respectively.
- Add each of the 0.5 g starch aliquots to 5 mL methanol in a 50 mL conical centrifuge tube, and fully disperse starch granules using several pulses of low intensity ultrasound (12–24 W/cm2) from an ultrasonic processor.
- Using a disposable transfer pipette, apply one small drop of the starch-methanol suspension (~0.2 mL) to the 100 mL of 50 g/L LiCl methanol electrolyte under constant stirring in the beaker. Close the sample compartment door.
- Perform a sizing run
- Click Preview in the bottom Instrument Toolbar to start a preview run. On the Status Panel, verify that the dynamically displayed concentration bar is in green, and shows a 5 to 8% nominal concentration range for the suspension.
- Click Stop on the bottom Toolbar to stop the Preview run. If necessary, dilute the starch-electrolyte suspension by replacing an aliquot of the suspension with the electrolyte, and then repeat a Preview run.
NOTE: The 5% to 8% nominal concentration range of the suspension is critical for completion of a run without stoppage due to aperture blockage by aggregated granules. If needed, adjust the drop-sample size, and/or the concentration of the starch-methanol suspension to make a new starch-electrolyte suspension having the nominal concentration in the optimal range. - After the verification, click Start on the bottom Toolbar to start the run. The analyzer automatically completes the run once the total count of sized granules, which is displayed along with the run time on the Status Panel in a run, reaches the set Total Count (125,000 or 250,000) by the Control Mode of the SOM. Depending on the suspension concentration (within 5-8% range or lower), a single run takes 2 to 5 min or more.
NOTE: When the analyzer automatically detects an aperture blockage per blockage detection settings of the SOM, it will abort the run, flush to unblock the aperture and start a new run. This blockage action is set to maximally repeat for four times before the analyzer cancels the run operation. This run-aborting blockage problem may be overcome by using two technical methods as noted in Table 2 and detailed in the discussion. - If needed, perform a technical repeat run (see Table 2 and detailed in Discussion) using the same starch-electrolyte suspension by simply clicking Start or Repeat on the bottom Toolbar.
- After completion of a run or repeat runs, empty the beaker, rinse it with methanol, and refill it with 100 mL fresh electrolyte solution for the next run.
- During a run, if an Extended Size Range notification dialog appears when the count of granules larger than 60 µm exceeds 0.1% of the total count (per the SOM setting), click Run 60% to 80% for running an extended dynamic sizing range to 80% of the aperture diameter.
NOTE: The Extended Size Range setting controls actions for granules larger than 60% of the aperture diameter (100 µm, in this case). The setting in the SOM specifies inclusion of starch granules larger than 60 µm when their counts reach over 0.1% of the total count. The completion of the run is still controlled by the total count, and may take slightly less time than otherwise without inclusion of the larger granules totaling less than 0.1% (presumed statically insignificant amount) of the total count.
- Analyze the run results
- If an SOM was used to control the runs, select Preferences settings as desired for viewing, printing, and statistical analyses of the results using the Create Preferences Wizard or the Edit Preferences under the SOP on the Main Menu.
- Overlay results from multiple runs on a single graph for comparison.
- Click Overlay on the Main Toolbar or File | Overlay on the Main Menu to access the Overlay window. Navigate to and select multiple desired result files in the Files box, click Add to move them to the Selected Files box, and click OK to overlay the selected results on a single graph.
- To add a file to an open overlay, click RunFile | Open for Overlay on the Run Menu to access the Overlay window, navigate to the desired file, and click to add.
- Average results from replicate analyses (2 extracts x 2 starch-sampling or 3 extracts x 1 starch-sampling), and view or print the average granule size distribution and statistics in a list or graph.
- On the Main Menu, click File | FileTool | Average to open the Average window. Navigate to and select multiple desired result files in the Files box, click Add to move them to the Selected Files box, and click OK to average the selected results and display the average on a single graph.
- To include an additional result file in an average distribution, on the Run Menu, click RunFile | Open and Add to Average to open the Add to Average window, navigate to and add the file. The new average appears on the graph in the Run (result) window or listing.
5. Specifying the average distribution
- In the Run-Menu window displaying the average distribution, click Calculate | Averaged Statistics on the Run Menu to open the statistics summary window, which displays the average statistics in rows, and the graph statistics for the average distribution in the columns.
- Use the graphic geometric mean (
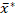 ) and S.D. (s*) in the graph statistics column to specify the average distribution in the
) and S.D. (s*) in the graph statistics column to specify the average distribution in the 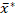 x/ s* form. Calculate the CV measuring variations among the averaged replicate distributions by dividing the mean (µ, the same as the
x/ s* form. Calculate the CV measuring variations among the averaged replicate distributions by dividing the mean (µ, the same as the 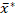 of the average distribution) of the geometric means of the averaged distributions with the average S.D. (σ) listed in the average statistics row.
of the average distribution) of the geometric means of the averaged distributions with the average S.D. (σ) listed in the average statistics row.
NOTE: The average S.D. (for µ) assessing variations among the means of the replicate distributions is different from the graphic geometric S.D. (for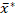 ) measuring the spread of the average distribution.
) measuring the spread of the average distribution.
Results
To validate the procedure, and demonstrate reproducibility of the determined granule size distribution, we performed replicate sizing analyses of sweetpotato starch samples. We prepared replicate (S1 and S2) starch samples from field-grown sweetpotatoes of a breeding line SC1149-19 at a similar developmental age using a previously described procedure28. From each starch extract, two 0.5 g aliquots (a and b) were sampled, suspended in 5 mL of methanol and sonicated with several pulses of low-energy...
Discussion
The outlined procedure has resolved some critical issues in several existing methods for starch granule size analyses, including inappropriate 1D or 2D sizing of 3D granules, distortion of sizing measurements due to none-uniform granule shapes, poor reproducibility and dubious statistical validity due to limited granule-sample sizes, inaccurate or improper specification (especially the use of the average size) of granule sizes in the presence of both granule shape and none-normal size distributions. It uses the ESZ techn...
Disclosures
The authors have nothing to disclose
Acknowledgements
This work is partly supported by the Cooperative Agriculture Research Center, and Integrated Food Security Research Center of the College of Agriculture and Human Sciences, Prairie View A&M University. We thank Hua Tian for his technical support.
Materials
| Name | Company | Catalog Number | Comments |
| Analytical beaker | Beckman Coulter Life Sciences | A35595 | Smart-Technology (ST) beaker |
| Aperture tube, 100 µm | Beckman Coulter Life Sciences | A36394 | For the MS4E |
| Disposable transfer pipettor, | Fisher Scientific (Fishersci.com) | 13-711-9AM | Other disposable transfer pipettors with similar orifice can also be used. |
| Fisherbrand Conical Polypropylene Centrifuge Tubes, 50 ml | Fisher Scientific (Fishersci.com) | 05-539-13 | Any other similar types of tubes can be used. |
| Glass beakers, 150 to 250 ml | Fisher Scientific (Fishersci.com) | 02-540K | These beakers are used to contain methanol for washing the aperture tube and stirrer between runs. |
| LiCl | Fisher Chemical | L121-100 | |
| Methanol | Fisher Chemical | A412-500 | Buy in bulk as the analysis uses a large quantity of methanol. |
| Mettler Toledo ML-T Precision Balances | Mettler Toledo | 30243412 | Any other precision balance with a readability 0.01 g to 1 mg will work. |
| Multisizer 4e Coulter Counter | Beckman Coulter Life Sciences | B23005 | The old model, Multisizer 3 can also be used with slight adjustment of parameters. The 4e model comes with a 100 μm aperture tube. Other aperture tubes of different diameter can be purchased separately from the company. |
| Ultrasonic processor UP50H | Hielscher Ultrasound Technology | UP50H | Other laboratory sonicator having a low-power (<50 Watt) output can be also used. Both MS1 and MS2 sonotrodes for the particular sonicator can be used to disperse starch granules in 5 ml methanol. Always use the lowest setting first, 20% amplitude and 0.1 or 0.2 cycle, and raise the setting if aggregates persist in suspension. |
References
- Shannon, J. C., Garwood, D. L., Boyer, C. D., BeMiller, J., Whistler, R. . Starch:Chemistry and Technology Food Science and Technology. , 23-82 (2009).
- Singh, N., Singh, J., Kaur, L., Singh Sodhi, N., Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chemistry. 81 (2), 219-231 (2003).
- Lindeboom, N., Chang, P. R., Tyler, R. T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch - Stärke. 56 (34), 89-99 (2004).
- Baldwin, P. M., Davies, M. C., Melia, C. D. Starch granule surface imaging using low-voltage scanning electron microscopy and atomic force microscopy. International Journal of Biological Macromolecules. 21 (1-2), 103-107 (1997).
- Jane, J. L., Kasemsuwan, T., Leas, S., Zobel, H., Robyt, J. F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke. 46 (4), 121-129 (1994).
- Matsushima, R., Nakamura, Y. . Starch: Metabolism and Structure. , 425-441 (2015).
- Wang, S. -. q., Wanf, L. -. l., Fan, W. -. h., Cao, H., Cao, B. -. s. Morphological analysis of common edible starch granules by scanning electron microscopy. Food Science. 32 (15), 74-79 (2011).
- Baldwin, P. M., Adler, J., Davies, M. C., Melia, C. D. Holes in starch granules: confocal, SEM and light microscopy studies of starch granule structure. Starch-Stärke. 46 (9), 341-346 (1994).
- Chakraborty, I., Pallen, S., Shetty, Y., Roy, N., Mazumder, N. Advanced microscopy techniques for revealing molecular structure of starch granules. Biophysical Reviews. 12 (1), 105-122 (2020).
- Bechtel, D. B., Wilson, J. D. Amyloplast formation and starch granule development in hard red winter wheat. Cereal Chemistry. 80 (2), 175-183 (2003).
- Evers, A. Scanning electron microscopy of wheat starch. III. Granule development in the endosperm. Starch-Stärke. 23 (5), 157-162 (1971).
- Wang, Y. J., White, P., Pollak, L., Jane, J. L. Characterization of starch structures of 17 maize endosperm mutant genotypes with Oh43 inbred line background. Cereal Chemistry. 70, 171-179 (1993).
- Peng, M., Gao, M., Abdel-Aal, E. S. M., Hucl, P., Chibbar, R. N. Separation and characterization of A-and B-type starch granules in wheat endosperm. Cereal Chemistry. 76, 375-379 (1999).
- Wilson, J. D., Bechtel, D. B., Todd, T. C., Seib, P. A. Measurement of wheat starch granule size distribution using image analysis and laser diffraction technology. Cereal Chemistry. 83 (3), 259-268 (2006).
- Liu, Q., Weber, E., Currie, V., Yada, R. Physicochemical properties of starches during potato growth. Carbohydrate Polymers. 51 (2), 213-221 (2003).
- Chmelik, J., et al. Comparison of size characterization of barley starch granules determined by electron and optical microscopy, low angle laser light scattering and gravitational field-flow fractionation. Journal of the Institute of Brewing. 107 (1), 11-17 (2001).
- Moon, M. H., Giddings, J. C. Rapid separation and measurement of particle size distribution of starch granules by sedimentation/steric field-flow fractionation. Journal of Food Science. 58 (5), 1166-1171 (1993).
- Wriedt, T., Wolfram, H., Wriedt, T. . The Mie Theory: Basics and Applications. , 53-71 (2012).
- Schuerman, D. W., Wang, R. T., Gustafson, B. &. #. 1. 9. 7. ;. S., Schaefer, R. W. Systematic studies of light scattering. 1: Particle shape. Applied Optics. 20 (23), 4039-4050 (1981).
- Goering, K. J., Fritts, D. H., Eslick, R. F. A study of starch granule size and distribution in 29 barley varieties. Starch-Stärke. 25 (9), 297-302 (1973).
- Chen, Z., Schols, H. A., Voragen, A. G. J. Starch granule size strongly determines starch noodle processing and noodle quality. Journal of Food Sciences. 68 (5), 1584-1589 (2003).
- Dai, Z. M. Starch granule size distribution in grains at different positions on the spike of wheat (Triticum aestivum L.). Starch-Starke. 61 (10), 582-589 (2009).
- Edwards, M. A., Osborne, B. G., Henry, R. J. Effect of endosperm starch granule size distribution on milling yield in hard wheat. Journal of Cereal Science. 48 (1), 180-192 (2008).
- Karlsson, R., Olered, R., Eliasson, A. C. Changes in starch granule size distribution and starch gelatinization properties during development and maturation of wheat, barley and rye. Starch - Starke. 35 (10), 335-340 (1983).
- Li, W. -. Y., et al. Comparison of starch granule size distribution between hard and soft wheat cultivars in Eastern China. Agricultural Sciences China. 7 (8), 907-914 (2008).
- Park, S. H., Wilson, J. D., Seabourn, B. W. Starch granule size distribution of hard red winter and hard red spring wheat: Its effects on mixing and breadmaking quality. Journal of Cereal Science. 49 (1), 98-105 (2009).
- Limpert, E., Stahel, W. A., Abbt, M. Log-normal distributions across the sciences: keys and clues. Bioscience. 51 (5), 341-352 (2001).
- Gao, M., et al. Self-preserving lognormal volume-size distributions of starch granules in developing sweetpotatoes and modulation of their scale parameters by a starch synthase II (SSII). Acta Physiologiae Plantarum. 38 (11), 259 (2016).
- Wattebled, F., et al. STA11, a Chlamydomonas reinhardtii locus required for normal starch granule biogenesis, encodes disproportionating enzyme. Further evidence for a function of alpha-1,4 glucanotransferases during starch granule biosynthesis in green algae. Plant Physiology. 132 (1), 137-145 (2003).
- Ji, Y., Seetharaman, K., White, P. J. Optimizing a Small-Scale Corn-Starch Extraction Method for Use in the Laboratory. Cereal Chemistry. 81 (1), 55-58 (2004).
- Halloy, S., Whigham, P. The lognormal as universal descriptor of unconstrained complex systems: a unifying theory for complexity. Proceedings of the 7th Asia-Pacific Complex Systems Conference. , 309-320 (2004).
- Furusawa, C., Suzuki, T., Kashiwagi, A., Yomo, T., Kaneko, K. Ubiquity of log-normal distributions in intra-cellular reaction dynamics. Biophysics (Nagoya-shi). 1, 25-31 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved