A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Bacterial Cellulose Spheres that Encapsulate Solid Materials
In This Article
Summary
This protocol presents an easy, inexpensive method of forming bacterial cellulose (BC) spheres. This biomaterial can function as an encapsulation medium for solid materials, including biochar, polymer spheres, and mine waste.
Abstract
Bacterial cellulose (BC) spheres have been increasingly researched since the popularization of BC as a novel material. This protocol presents an affordable and simple method for BC sphere production. In addition to producing these spheres, an encapsulation method for solid particles has also been identified. To produce BC spheres, water, black tea, sugar, vinegar, and bacterial culture are combined in a baffled flask and the contents are agitated. After determining the proper culture conditions for BC sphere formation, their ability to encapsulate solid particles was tested using biochar, polymer beads, and mine waste. Spheres were characterized using ImageJ software and thermal gravimetric analysis (TGA). Results indicate that spheres with 7.5 mm diameters can be made in 7 days. Adding various particles increases the average size range of the BC capsules. The spheres encapsulated 10 - 20% of their dry mass. This method shows low-cost sphere production and encapsulation that is possible with easily obtainable materials. BC spheres may be used in the future as a contaminant removal aid, controlled release fertilizer coating, or soil amendment.
Introduction
Bacterial cellulose (BC) has been noted for its potential industry use due to its mechanical strength, high purity and crystallinity, water retention abilities, and intricate fiber structure1,2,3,4. These characteristics make BC a favorable biomaterial for a variety of applications, including biomedical, food processing, and environmental remediation uses1. Formation of a BC film can be done with single organism cultures or mixed cultures like those used for kombucha5, a fermented tea beverage. Brewing kombucha relies on a "Symbiotic Culture Of Bacteria and Yeast", commonly known as a SCOBY. Using this symbiotic culture of organisms, a similar technique is employed to create BC spheres. This biomaterial may be employed to help isolate environmental contaminants and anchor agricultural amendments like biochar to achieve more efficient crop production.
Previous literature has discussed how the characteristics of BC produced in agitated conditions compare to BC produced in a stationary culture. A stationary culture results in a film that forms at the liquid-air interface, while a shaken culture results in varying BC particles, strands, and spheres suspended within the liquid6. Many studies have referenced the claim that commercial production of BC is more feasible in the dynamic conditions6,7, providing rationale for applying this paper's method. Additionally, various investigations on the structure and properties of BC spheres have been conducted. Toyosaki et al.6 compared baffled and smooth-walled Erlenmeyer flasks in their agitated BC production. A study by Hu and Catchmark4 determined conditions for BC spheres that were used as guidelines for the current BC sphere production process, and their results indicate that the sphere size does not continue to increase after 60 hours. A review of BC production by Mohammad et al.1 indicates that shaking the BC culture ensures even oxygen supply and distribution, which is necessary for successful BC growth. Holland et al.8 have studied the crystallinity and chemical structure of BC using X-ray diffraction and Fourier transform infrared spectroscopy. It is assumed BC capsules will exhibit similar characteristics and future research will investigate structural properties. Studies have also explored the beneficial effects of using BC to produce improved biocomposites. Using epoxy-resin as a base, researchers have showed that the addition of BC improves material characteristics like fatigue life, fracture toughness, and tensile and flexural strength9,10. As shown by past and current research, many are interested in commercializing BC use.
Many researchers have investigated bacterial cellulose in controlled release systems, and the method described here generates capsules that could be utilized as controlled release systems. Much of this research focuses on controlled release in the biomedical field, as well as some exploration in controlled release fertilizer (CRF) administration. Based on the success of BC's controlled release of amoxicillin11, lidocaine12, and ibuprofen13, BC may exhibit similar delivery characteristics with other substances, such as a pelletized fertilizer. An overview of CRF's by Shaviv and Mikkelsen14 acknowledges that CRF's are more efficient, save labor, and generally cause less environmental degradation than conventional fertilizer application. Bacterial cellulose may work as a favorable encapsulating material for CRF's. Fertilizers may leach out of BC membranes or discharge as BC biodegrades15,16. BC's high water swelling capacity can also act as a beneficial soil amendment17,18,19 because both fertilizer nutrients and moisture may release into the ground through application of BC spheres. With these traits, a CRF formed by BC sphere encapsulation may have an advantage over other fertilizer coating materials that could have negative effects during their production and disposal stages. Adapting BC into a fertilizer coating may further improve CRF technologies. By lowering fertilizer release rate, crops will have sufficient time to uptake the fertilizer and prevent excess runoff into bodies of water, thereby reducing eutrophication and unoxygenated zones. Similar slow-release fertilizers have been prepared and piloted using polymer coatings20.
Unlike protocols outlined in previous research, this one focuses on uniform, cohesive sphere production rather than high cellulose yield. Moreover, BC encapsulation of other solids has been studied with cellulose films, but not spheres21. By expanding the research on bacterial cellulose spheres, further steps can be made to produce BC commercially, which is beneficial because of BC's environmentally safe features. This method of BC sphere fabrication utilizes inexpensive, readily available culinary ingredients. After the initial assembly, BC spheres begin to form within 2 days without interference. Producing BC spheres through this strategy requires little space and has an edible by-product, the fermented tea 'kombucha'. Encapsulation techniques mentioned in other studies include coatings formed through the phase inversion technique22,23, matrix formation24, spray drying25, and direct encapsulation during synthesis26. The direct encapsulation method outlined in this manuscript is useful for those who desire an easy, inexpensive process that utilizes readily available materials.
Through this research, a successful protocol for BC sphere production and encapsulation was created. BC spheres can encapsulate solid particles of biochar, mine tailings, and polystyrene microbeads within their individual structures. Although not widely used in industry yet, BC is a practical, sustainably made, and naturally occurring material that could be used for future applications.
Protocol
1. Creation and maintenance of bacterial cellulose starter culture
- Obtain a starter culture of bacterial cellulose, approximately 50 g, in the form of a SCOBY. It can be purchased commercially (e.g., from Cultures for Health). Place the SCOBY into a 1 L beaker, covered with a paper towel.
- Boil 700 mL of deionized water, transfer it to a separate vessel from the one containing the SCOBY, and add 85 g of sucrose.
- Once the sucrose has dissolved, add 2 bags of black tea (4.87 g). Steep the tea for 1 h, then carefully remove the tea bags using a stir rod.
- Add 200 mL of distilled white vinegar to the tea. Let the mixture cool to 25 °C. Once cooled, add 700 mL of the room temperature tea to the beaker containing the SCOBY.
CAUTION: Adding the acidic tea while it is too hot may harm the organisms in the SCOBY. - Cover the beaker with a paper towel and secure it with an elastic band, and place the beaker in a storage area that maintains a temperature of 25 °C. This vessel is commonly referred to as stock culture or a hotel.
- To keep the SCOBY healthy, maintenance is required about 2 times a month.
- Using gloved hands to hold back the SCOBY mats, drain the liquid from the hotel into a separate beaker. In the container with the liquid, add enough acidic tea for a total of 700 mL of solution.
- Dissolve 65 g of sucrose in the container with the acidic tea. While waiting for the sucrose to dissolve, carefully rinse the SCOBY mats in DI water.
- Once the sucrose is fully dissolved, the liquid can be added to the beaker containing the rinsed SCOBY mats. Cover the beaker and return it to the incubation area.
2. Production of bacterial cellulose spheres
NOTE: Exercise caution when working with boiling water. Ensure glassware can withstand the boiling water temperatures, is free of defects, and is the appropriate size. A schematic describing the production of BC spheres is given in Figure 1.
- Boil 350 mL of deionized water using a tea kettle. Transfer the hot water to a 500 mL beaker. Dissolve 42.5 g of granulated sucrose into the hot water using a stir rod.
- When the sucrose is fully dissolved, steep 1 bag of black tea (2.54 g) in the flask containing the sucrose and water for 1 h. After this, remove the tea bag with a stir rod, taking care to avoid breaking open the tea bag, and then dispose of it in the trash.
- Add 100 mL of distilled white vinegar to the beaker, and then thoroughly stir the mixture. Transfer 80 mL of the acidic tea mixture to a 250 mL baffled flask. Allow the tea mixture to cool to room temperature, 20 - 25 °C.
NOTE: At this point, the mixture can be left to cool overnight or until prepared for the next step. - Once the liquid temperature is at room temperature (20 - 25 °C), add 20 mL of microbial starter culture liquid to the baffled flask. This liquid can be obtained from a SCOBY hotel. Cover the flask with parafilm.
- Place the baffled flask on an orbital shake table and set the speed to 125 rotations per minute (rpm). Allow the mixture to shake for 3 days in a room or incubator with a temperature from 20 - 25 °C to produce BC spheres.
NOTE: If irregular shapes form in the flask contents or if cellulose clumps stick to the walls of the flask, they should be removed to prevent further irregular BC masses from forming. Use tweezers to remove unwanted BC masses, including thin strings, rings, tubular shapes, and other clearly non-spherical shapes. - Once the spheres have formed, gently pour them from the flask and analyze, dispose, or use them in a way not outlined in this paper.
3. Using bacterial cellulose spheres to encapsulate particles or contaminants
- Follow steps 2.1-2.5 above.
- After shaking for 3 days, add about 0.01 g of finely ground particulate matter to the baffled flask. Appropriate solids include biochar (260 ± 140 µm), mine waste (350 ± 140 µm), and polystyrene microbeads (3 µm). Data for these materials are outlined in the Representative Results section. Please see the attached Table of Materials for further descriptions of biochar, mine waste, and microbeads.
- Cover the flask again with the parafilm and place it back on an orbital shaker, using the same speed and ambient temperature (20 - 25 °C) for 3 more days. Remove the BC encapsulated particles for analysis, disposal, or other uses.
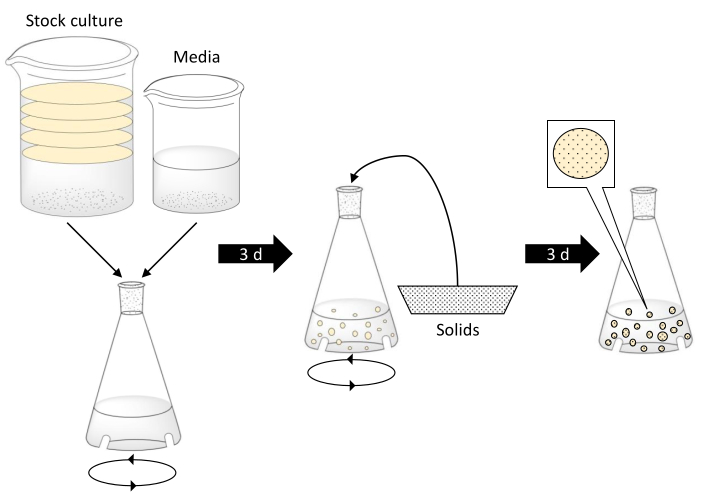
Figure 1. Bacterial cellulose sphere fabrication and encapsulation of solid particles. Step 1 involves combining bacterial stock culture with black tea, sugar, and vinegar media in a baffled flask. The disks in the stock culture represent BC mats. Then, the baffled flask is placed on an orbital shake table for 3 days. The middle step shows solids being added to the flask once BC spheres have formed. The flask is shaken for 3 more days. In the final step, BC spheres have continued increasing in size and encapsulated the solid particles. Please click here to view a larger version of this figure.
Results
BC spheres have the fastest growth rate during the first 48 h of culture (Figure 2). Figure 2 also shows how the spheres tend to reach a maximum average size and then remain constant. In this experiment, the spheres reached an average diameter of 7.5 ± 0.2 mm. Although the BC spheres never completely deteriorate within the 10 day growth period, they did start to form tendrils that extend off the main body of the sphere around the eighth day. This can be see...
Discussion
This protocol outlines BC sphere production and encapsulation methods that are easy to conduct and cost effective. Through various adjustments to the original protocol, an adequate process has been identified. Critical steps must be followed to ensure viable spheres. All the ingredients involved in BC formation play a key role in the health and durability of the spheres. The sucrose feeds organisms, the tea provides nitrogen, and the vinegar lowers the pH to optimal conditions to prevent undesired contaminants
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is a continuation of a Montana Tech Research Assistant Mentorship Program project by Adolfo Martinez, Catherine Mulholland, Tyler Somerville, and Laurel Bitterman. Research was sponsored by the National Science Foundation under Grant No. OIA-1757351 and the Combat Capabilities Development Command Army Research Laboratory (Cooperative Agreement Number W911NF-15-2-0020). Any opinions, findings and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the Army Research Lab. We would also like to thank Amy Kuenzi, Lee Richards, Katelyn Alley, Chris Gammons, Max Wohlgenant, and Kris Bosch for their contributions.
Materials
| Name | Company | Catalog Number | Comments |
| 100 mL graduated cylinder | |||
| 1000 mL beaker | |||
| 25 mL graduated cylinder | |||
| 250 mL Erlenmeyer baffled flask | Chemglass | CLS-2040-02 | |
| 500 mL beaker | |||
| Balance | |||
| Biochar | Ponderosa pine heat treated under argon gas, heated at 15 °C per minute to 800 °C | ||
| Black tea | |||
| Deionized water | |||
| Distilled white vinegar | |||
| Elastic band | |||
| Microbial starter culture | Cultures for Health | ||
| Mine waste | Collected from Butte, MT: 46.001978,-112.582465. Mine waste contains soil and metals originating from past copper mining. Mn, Si, Ca, Al, and Fe were the five most prevalent elements measured in the mine waste through x-ray diffraction. | ||
| Mortar and pestle | |||
| Orbital shaker | Used various brands | ||
| Paper towel | |||
| Polystyrene microbeads | Polybead | 17138 | 3 micron diameter |
| Stir rod | |||
| Sucrose | |||
| Tea kettle | |||
| TGA | TA Instruments | TA Q500 | 400 °C/min to 800 °C, 100 mL/min N2 |
| Thermometer | |||
| XRF Analyzer | ThermoFisher Scientific | 10131166 |
References
- Mohainin Mohammad, S., Abd Rahman, N., Sahaid Khalil, M., Rozaimah Sheikh Abdullah, S. An Overview of Biocellulose Production Using Acetobacter xylinum Culture. Advances in Biological Research. 8 (6), 307-313 (2014).
- Dufresne, A. Bacterial cellulose. Nanocellulose. , 125-146 (2017).
- Czaja, W., Romanovicz, D., Brown, R. M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose. 11 (3-4), 403-411 (2004).
- Hu, Y., Catchmark, J. M. Formation and characterization of spherelike bacterial cellulose particles produced by acetobacter xylinum JCM 9730 strain. Biomacromolecules. 11 (7), 1727-1734 (2010).
- Goh, W. N., Rosma, A., Kaur, B., Fazilah, A., Karim, A. A., Bhat, R. Microstructure and physical properties of microbial cellulose produced during fermentation of black tea broth (kombucha). International Food Research Journal. 19 (1), 153-158 (2012).
- Toyosaki, H., Naritomi, T., Seto, A., Matsuoka, M., Tsuchida, T., Yoshinaga, F. Screening of Bacterial Cellulose-producing Acetobacter Strains Suitable for Agitated Culture. Bioscience, Biotechnology, and Biochemistry. 59 (8), 1498-1502 (1995).
- Shi, Z., Zhang, Y., Phillips, G. O., Yang, G. Utilization of bacterial cellulose in food. Food Hydrocolloids. 35, 539-545 (2014).
- Holland, M. C., Eggensperger, C. G., Giagnorio, M., Schiffman, J. D., Tiraferri, A., Zodrow, K. R. Facile Postprocessing Alters the Permeability and Selectivity of Microbial Cellulose Ultrafiltration Membranes. Environmental Science and Technology. 54 (20), 13249-13256 (2020).
- Le Hoang, S., Vu, C. M., Pham, L. T., Choi, H. J. Preparation and physical characteristics of epoxy resin/ bacterial cellulose biocomposites. Polymer Bulletin. 75 (6), 2607-2625 (2018).
- Vu, C. M., Nguyen, D. D., Sinh, L. H., Pham, T. D., Pham, L. T., Choi, H. J. Environmentally benign green composites based on epoxy resin/bacterial cellulose reinforced glass fiber: Fabrication and mechanical characteristics. Polymer Testing. 61, 150-161 (2017).
- Pavaloiu, R. D., Stoica, A., Stroescu, M., Dobre, T. Controlled release of amoxicillin from bacterial cellulose membranes. Central European Journal of Chemistry. 12 (9), 962-967 (2014).
- Trovatti, E., et al. Biocellulose membranes as supports for dermal release of lidocaine. Biomacromolecules. 12 (11), 4162-4168 (2011).
- Trovatti, E., et al. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. International Journal of Pharmaceutics. 435 (1), 83-87 (2012).
- Shaviv, A., Mikkelsen, R. L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation - A review. Fertilizer Research. 35 (1-2), 1-12 (1993).
- Eggensperger, C. G., et al. Sustainable living filtration membranes. Environmental Science and Technology Letters. 7 (3), 213-218 (2020).
- Schröpfer, S. B., et al. Biodegradation evaluation of bacterial cellulose, vegetable cellulose and poly (3-hydroxybutyrate) in soil. Polimeros. 25 (2), 154-160 (2015).
- Orts, W. J., Glenn, G. M. Reducing soil erosion losses with small applications of biopolymers. ACS Symposium Series. 723, 235-247 (1999).
- Mohite, B. V., Patil, S. V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnology and Applied Biochemistry. 61 (2), 101-110 (2014).
- Mikkelsen, R. L. Using hydrophilic polymers to control nutrient release. Fertilizer Research. 38 (1), 53-59 (1994).
- Du, C. W., Zhou, J. M., Shaviv, A. Release characteristics of nutrients from polymer-coated compound controlled release fertilizers. Journal of Polymers and the Environment. 14 (3), 223-230 (2006).
- Serafica, G., Mormino, R., Bungay, H. Inclusion of solid particles in bacterial cellulose. Applied Microbiology and Biotechnology. 58 (6), 756-760 (2002).
- Tomaszewska, M., Jarosiewicz, A. Use of polysulfone in controlled-release NPK fertilizer formulations. Journal of Agricultural and Food Chemistry. 50 (16), 4634-4639 (2002).
- González, M. E., et al. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Science of the Total Environment. 505, 446-453 (2015).
- Shavit, U., Shaviv, A., Shalit, G., Zaslavsky, D. Release characteristics of a new controlled release fertilizer. Journal of Controlled Release. 43 (2-3), 131-138 (1997).
- Kolakovic, R., Laaksonen, T., Peltonen, L., Laukkanen, A., Hirvonen, J. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. International Journal of Pharmaceutics. 430 (1-2), 47-55 (2012).
- Zaharia, A., et al. Bacterial cellulose-poly(acrylic acid-: Co-N, N ′-methylene-bis-acrylamide) interpenetrated networks for the controlled release of fertilizers. RSC Advances. 8 (32), 17635-17644 (2018).
- Peterson, J. D., Vyazovkin, S., Wight, C. A. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly(propylene). Macromolecular Chemistry and Physics. 202 (6), 775-784 (2001).
- Goh, W. N., Rosma, A., Kaur, B., Fazilah, A., Karim, A. A., Bhat, R. Fermentation of black tea broth (kombucha): I. effects of sucrose concentration and fermentation time on the yield of microbial cellulose. International Food Research Journal. 19 (1), 109-117 (2012).
- Zhu, H., Jia, S., Yang, H., Jia, Y., Yan, L., Li, J. Preparation and application of bacterial cellulose sphere: A novel biomaterial. Biotechnology and Biotechnological Equipment. 25 (1), 2233-2236 (2011).
- Nguyen, V. T., Flanagan, B., Gidley, M. J., Dykes, G. A. Characterization of cellulose production by a Gluconacetobacter xylinus strain from Kombucha. Current Microbiology. 57 (5), 449-453 (2008).
- Costa, A. F. S., Almeida, F. C. G., Vinhas, G. M., Sarubbo, L. A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Frontiers in Microbiology. 8, 1-12 (2017).
- Watanabe, K., Tabuchi, M., Morinaga, Y., Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose. 5 (3), 187-200 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved