Method Article
Production of Recombinant PRMT Proteins using the Baculovirus Expression Vector System
In This Article
Summary
The baculovirus expression vector system (BEVS) is a robust platform for expression screening and production of protein arginine methyltransferases (PRMTs) to be used for biochemical, biophysical, and structural studies. Milligram quantities of material can be produced for the majority of PRMTs and other proteins of interest requiring a eukaryotic expression platform.
Abstract
Protein arginine methyltransferases (PRMTs) methylate arginine residues on a wide variety of proteins that play roles in numerous cellular processes. PRMTs can either mono- or dimethylate arginine guanidino groups symmetrically or asymmetrically. The enzymology of these proteins is a complex and intensely investigated area that requires milligram quantities of high-quality recombinant protein. The baculovirus expression vector system (BEVS) employing Autographa californica multiple nucleopolyhedrovirus (AcMNPV) and Spodoptera frugiperda 9 (Sf9) insect cells has been used for expression screening and production of many PRMTs, including PRMT 1, 2, and 4 through 9. To simultaneously screen for the expression of multiple constructs of these proteins, including domains and truncated fragments as well as the full-length proteins, we have applied scalable methods utilizing adjustable and programmable multichannel pipettes, combined with 24- and 96-well plates and blocks. Overall, these method adjustments enabled a large-scale generation of bacmid DNA, recombinant viruses, and protein expression screening. Using culture vessels with a high-fill volume of Sf9 cell suspension helped to overcome space limitations in the production pipeline for single batch large-scale protein production. Here, we describe detailed protocols for the efficient and cost-effective expression of functional PRMTs for biochemical, biophysical, and structural studies.
Introduction
Protein arginine methyltransferases (PRMTs) methylate arginine residues in a monomethyl or symmetric/asymmetric dimethyl fashion. The repetitive RG/RGG/GRG sequences are highly preferred by most PRMTs and are found in a wide variety of proteins1,2. Arginine methylated proteins such as histones or transcription factors and splicing factors regulate transcription, splicing, and chromatin structure3,4. Increasing knowledge of diverse regulation of substrate and cofactor utilization, turnover, and kinetics of PRMTs, as well as generation of selective inhibitors, have shed mechanistic light on these enzymes and their complexes5,6. However, not all PRMT family members are studied to the same extent; for example, PRMT9 was only recently discovered to be a member of the PRMT family1. Structure and enzyme function studies for these proteins require sufficient, often milligram, quantities of recombinant protein to be available.
The Escherichia coli (E. coli) prokaryotic expression system is usually the first choice for expression screening utilizing multiple constructs for a given protein7,8,9. However, E. coli-based expression does not always result in sufficient quantities of PRMT proteins in their active forms, as we have noted in particular for PRMT5 and PRMT7 (see below). Thus, PRMTs that failed to express in E. coli or needed to be produced by the eukaryotic expression machinery were subcloned into vectors appropriate for the expression screening in the alternative baculovirus expression vector system (BEVS). While E. coli expressed samples of PRMT1, PRMT3 and PRMT8 have been utilized extensively for in vitro assays and crystallography, other PRMTs such as PRMT5, which requires MEP50 binding partner of its dual methyltransferase domain, and PRMTs such as PRMT7 and 9, necessitate insect cell expression to obtain sufficient quantities of active protein. Overall, the standardized medium-throughput methyltransferase assays for PRMT4, 5, 6, 7, and 9 have utilized the BEVS in insect cells6. The baculovirus expression vector system (BEVS) is a versatile platform to produce recombinant proteins requiring the eukaryotic expression machinery that enables post-translational modifications essential for biochemical, biophysical, and structural studies10,11,12. Several BEVSs have become commercially available since the first reported use of baculoviruses in 1983 for protein expression13. Most of these protocols employ different strategies for the transfer of the expression plasmid into insect cells. These include Bac-to-Bac, flashBAC, BaculoGOLD Bright, BacVector-3000, BacMagic, BacPAK, etc. Our protocol is based on the most commonly used system in BEVS, the Bac-to-Bac system14, which is designed to transfer the gene/cDNA encoding the protein of interest (POI, here the PRMTs) into the baculovirus genome maintained in a specialized strain of E. coli via site-specific transposition15.
Briefly, the plasmid transfer vector containing the gene of interest was transformed into DH10Bac E. coli competent cells to generate recombinant viral bacmid DNA. Adherent Sf9 cells were then transfected with bacmid DNA. Four to five days after transfection, initial recombinant baculoviruses secreted into the cell culture medium were recovered and labeled as the P1 virus. The P1 baculovirus stocks were then used for virus amplification (i.e., generation of P2 baculovirus stocks) and protein expression screening. Based on the expression screening results, P2 viruses for the best expression construct of the protein were identified and used to generate suspension cultures of baculovirus-infected insect cells (SCBIIS) for the large-scale protein production. Here, we describe our detailed protocols and describe the rationale behind our reagent and culture vessel choices to support our strategy of developing a more time-efficient, cost-efficient, and scalable methodology to obtain sufficient quantities of desired recombinant proteins.
Protocol
NOTE: The overview of the BEVS protocol steps is outlined in Figure 1.
1. Generation of a recombinant bacmid DNA
- Preparation of LB agar selective plates for DH10Bac transformation
- Prepare LB agar plates containing: 50 µg/mL kanamycin, 7 µg/mL gentamicin, 10 µg/mL tetracycline, 200 µg/mL Bluo-gal, and 40 µg/mL IPTG to select for DH10Bac transformants.
- Weigh 25 g of premixed LB broth and 13 g of Bacto Agar and put into 2 L flask. Bring the volume to 1 L with distilled water and autoclave for 15-30 min at 121 °C.
- Set a water bath at 50 °C and cool the autoclaved agar solution in the water bath for 40- 60 min until it is cooled down to 50-55 °C.
- To the cooled solution, add gentamicin to a final concentration of 7 µg/mL, kanamycin to a final concentration of 50 µg/mL, tetracycline to a final concentration of 10 µg/mL, Bluo-gal to a final concentration of 200 µg/mL, and IPTG to a final concentration of 40 µg/mL.
- Mix the agar solution and aliquot 7-10 mL of the medium to each 60 mm plate using a 50 mL pipette. Let the plates sit at room temperature (2 h) to harden. Invert, wrap and store at 4 °C. Plates containing antibiotics are stable for up to 4 weeks.
- Transformation of the plasmid into DH10Bac E. coli competent cells
- Calculate the required volume of competent cells.
- Thaw competent cells on ice, gently spin down and resuspend back by gentle tapping.
- Use a 12-channel pipette to dispense 4 µL of the cells into each well of the 96-well PCR plate.
- Add ~ 0.3-0.5 µg of recombinant plasmid DNA to the competent cells and mix gently by tapping. Incubate the mixture on ice for 10-15 min.
- Heat-shock the mixture in a PCR machine at 42 °C for 45 s. Chill on ice for 2 min.
- Dispense 0.5 mL of SOC medium to each well of the 96-well blocks (96-deep well 2.4 mL).
- Use a 12-channel pipette to transfer the transformed bacterial suspension into the corresponding well of the block and cover it with an airpore sheet.
- Place the block in a shaking incubator at 37 °C with medium agitation (205 rpm) for 4-5 h.
- Evenly spread 30-50 µL of the culture over the surface of the LB agar plate using sterile glass beads. Store the rest of the culture at 4 °C.
- Incubate the plates at 37 °C until the color of the blue/white colonies is discernible (40-48 h). Discard the culture when enough white, large colonies are obtained on the plate.
- To ensure that white colonies contain only recombinant bacmid DNA, re-streak one isolated white colony onto fresh LB agar plates containing antibiotics, bluo-gal, and IPTG to verify the phenotype.
- Incubate for 48 h at 37 °C.
- Pick one verified white colony from a re-streaked plate for the extraction of recombinant bacmid DNA.
- Isolating recombinant bacmid DNA
- Inoculate a single, isolated white colony into 3 mL of LB medium supplemented with 50 µg/mL kanamycin, 7 µg/mL gentamicin, and 10 µg/mL tetracycline in 24-well blocks (24-well blocks round bottom) and cover with an airpore sheet.
- Grow at 37 °C overnight with shaking at 250 rpm.
- Centrifuge the 24-well blocks at 2,100 x g for 10 min. Decant the supernatant, invert the block, and tap gently on an absorbent tissue paper. Add 250 µL of solution 1 (cell resuspension solution) to each well.
- Seal the blocks with tape pads or any other sealing films and place them onto a shaking platform at 75 rpm for 5-10 min. Check each well to ensure proper cell lysis, and resuspend if necessary, using a 1 mL tip.
- Add 250 µL of solution 2 (cell lysis solution) to each well, seal the blocks, and place onto a shaking platform at 75 rpm for 30 s (to avoid cross-contamination of the samples, do not invert 24-well blocks) incubating at room temperature for 4 min.
- Add 250 µL of solution 3 (neutralization solution), seal the blocks, and place onto the shaking platform at 75 rpm for 30 s. A thick white precipitate of protein and E. coli genomic DNA will form. Place the sample on ice for 10-15 min.
- Centrifuge for 60 min at 2,100 x g at 4 °C to tightly pellet the white precipitate material. During centrifugation, label a new microcentrifuge tube and add 0.8 mL of absolute isopropanol.
- Gently transfer the supernatant to the tube containing isopropanol, avoiding any white precipitate material. Mix by gently inverting the tube a few times and then place on ice for 5 to 10 min. At this stage, the sample can be stored at -20 °C overnight.
- Centrifuge the sample for 15 min at 14,000 x g at 4 °C. Remove the supernatant and add 0.5 mL of 70% ethanol to each tube. Invert the tube several times to wash the pellet. Centrifuge for 5 min at 14,000 x g at room temperature. (Optional: repeat wash)
- Bring samples inside the laminar flow hood to ensure the plasmid preparation's sterility and remove as much of the supernatant as possible.
NOTE: The pellet may become dislodged from the bottom of the tube, so carefully watch the pellet when discarding the supernatant. - Air-dry the pellet inside the laminar flow hood for 15 - 20 min and dissolve the DNA in 50 µL of filtered elution buffer, 10 mM Tris-Cl, pH 8.5 (make sure that pellets are not over-dried).
- Since recombinant bacmid DNA is greater than 135 kb in size, to avoid shearing of DNA, dissolve the pellet by gentle tapping.
- To verify the presence of the gene of interest in bacmid DNA, set up the PCR reaction mix in a 96-well PCR plate.
- Prepare the PCR mix as follows: 1 µL of buffer, 0.2 µL (10 mM each) of dNTPs, 0.1 µL of Taq DNA polymerase, 0.1 µL (25 µM) of forward and reverse primers (BACV2FWD: tattccggattattcataccg; BACV2REV: ctctacaaatgtggtatggc), 1 µL of bacmid DNA and 8 µL of water.
- Perform amplification of the target genes with initial denaturation for 2 min at 95 °C, followed by 25 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min/kb.
- Terminate the reaction after a final extension for 7 min at 72 °C.
- Analyze 10 µL of the amplification product on 1% (w/v) agarose gel containing nucleic acid staining solution by electrophoresis.
- Store the verified bacmid DNAs at 4 °C.
2. Generation of recombinant baculovirus stocks
NOTE: Use exponentially growing Sf9 cells with a viability of 95% or greater for any step of the baculovirus expression protocol, including cell transfection for baculovirus generation, baculovirus volume amplification, protein expression screening, and protein production.
- Transfection of Sf9 cells with Bacmid DNA and transfection reagents (T.R.) such as JetPrime or X-tremeGENE 9.
NOTE: Trypan Blue staining and a hemocytometer can be used to determine viable cell counts and % cell viability. Non-viable cells take up the stain and appear blue under the microscope while viable cells remain unstained. To calculate % cell viability, a total cell count is obtained (unstained and stained) and the viable cell count is divided by the total cell count and multiplied by 100.- Dilute the exponentially growing Sf9 cells to a final cell density of 4 x 105 cells/mL in serum free insect media and pour into the sterile reagent reservoir.
- Use a programmable multichannel pipette to seed 0.5 mL of the diluted Sf9 cells into each well of a 24-well plate.
- Label one well of the plate as a control (untransfected) and use it as a control to compare the transfected and non-transfected cells to assess for potential signs of infections.
- After seeding the cells into the plates, gently rock the plates back and forth several times to ensure an even monolayer of cells. Do not swirl the plates because the cells will cluster into the center of the well.
- Incubate the plates at 27 °C for at least 1 h to allow for cell attachment to the culture plates.
- Mix well the transfection reagent vial. For each transfection, add 2 µL of the transfection reagent to 100 µL of the transfection buffer. Any other unsupplemented insect medium can also be used. Deposit the diluted transfection reagent in a sterile reagent reservoir and gently mix for 10 s.
- Using a 12-channel pipette, transfer 102 µL of the diluted transfection reagent into a sterile 96-microwell plate.
- Transfer 10 µL of a 0.2 µg/µL solution of recombinant bacmid DNA into the corresponding well of a 96-well microwell plate and mix by gently shaking (tapping) the plate from the sides.
- Incubate the transfection mixture for 15-20 min to enable complex formation.
- Using an adjustable 6-channel pipette designed for transfer between 96- and 24-well plates, add the transfection mix onto the cells dropwise into corresponding wells of transfection plates and incubate for 4-5 h at 27 °C.
- Gently rock the plates back and forth several times during the incubation time to ensure the even distribution of the transfection mixture over the cell monolayer.
- 4-5 h after transfection, add 1.5 mL of insect serum-free medium supplemented with 10% (v/v) final of heat inactivated fetal bovine serum and antibiotic-antimycotic to 1% (v/v) final volume (100 units/mL of penicillin, 100 µg/mL of streptomycin, and 0.25 µg/mL of amphotericin B).
- Incubate the cells in a 27 °C incubator for 72-96 h. Gently rock the transfection plates once a day when possible.
- Look for signs of infection (SOI), evident in transfected cells at 72-96 h post-transfection (Figure 2). Keep in mind that the transfected cells will start producing the virus and further infect the culture; thus, look for the signs of infection.
NOTE: Signs of infection are structural changes in the insect cells, such as a 25-50% increase in the cell diameter, enlarged cell nuclei, uniformly rounded shape, loss of proliferation and adherence to the culture dish surface, as well as a decrease in cell viability (Figure 2. Baculovirus Infected and uninfected Sf9 cells).
3. Small - scale protein expression screening and virus amplification
- Infection of Sf9 cells with P1 baculovirus stocks.
NOTE: 4-5 days after transfection, signs of infection should be evident in the transfected cells when compared to the control (untransfected) cells under an inverted microscope. The initial recombinant baculoviruses secreted into the cell culture medium should be ready to collect.- Seed 2 x 105 exponentially growing Sf9 cells in serum-free insect media into each well of the 24-well plates in a total volume of 2 mL for infection with P1 viruses to amplify virus volume (resulting in the generation of the P2 viruses).
- After pipetting the cells into the plates, gently rock the plates, using back and forth motion to ensure an even monolayer. Do not swirl the plates because the cells will cluster into the center of the well.
- Incubate the plates at 27 °C for at least 1 h to allow for cellular adherence to the plate.
- Dispense 4 mL of Sf9 cells at a density of 3.5-4 x 106 in insect serum-free medium into each well of 24-well blocks to infect with P1 viruses for the protein expression screening.
NOTE: Label one well of the 24-well plates and 24-well blocks as control and use as an uninfected control for comparison of infected and non-infected cells when looking for SOI. - Use a programmable electronic multichannel to allow the simultaneous collection of P1 viruses (step 2.1.13), infection of freshly seeded Sf9 cells (step 3.1.1) and infection of the suspension cells in the 24-well blocks (step 3.1.4) with 150 µL of P1 viruses.
- Spin down the rest of the collected P1 viral stocks for 15 min at 17,970 x g, transfer them into microcentrifuge tubes and store in the dark at 4 °C.
- Gently rock the 24-well plates (step 3.1.1) on a reciprocating shaker to ensure an even distribution of the added P1 viruses over the cell monolayer; repeat this a few times during the incubation time.
- Cover the 24-well blocks with the suspension culture of infected Sf9 cells (step 3.1.4) with an airpore sheet.
- Incubate the 24-well blocks at 27 °C, shaking at 245 rpm for 72-96 h.
- Look for SOI within 72-96 h after infection time in the 24-well plates with P2 viruses (step 3.1.1) and in the 24-well blocks (step 3.1.4) with infected cells for the expression screening.
NOTE: 4-5 days after infection of Sf9 cells with P1 viruses, SOI should be evident in the infected cells when compared to the non-infected control cells under an inverted microscope. - Collect P2 viruses from 24-well plates, centrifuge for 15 min at 17,970 x g, transfer them into microcentrifuge tubes and store in the dark at 4 °C.
- At 72-96 h post-infection, spray over the 24-well blocks with 70% ethanol, bring into the laminar flow hood, and check the cell density and viability in a few wells by Trypan Blue staining.
- Proceed to protein purification if cells have signs of infection and viability is close to 70-75% as assessed by Trypan Blue staining.
- Pellet the cells by centrifuging the 24-well blocks at 525 x g at 4 °C for 15 min. Discard the supernatant and thoroughly re-suspend the pellets in 1 mL of lysis buffer comprising 25 mM Tris pH 8.0, 300 mM NaCl, 0.6 % NP-40, 2 mM imidazole, 5% glycerol (v/v) and 1x protease inhibitor cocktail (100x protease inhibitor cocktail comprises aprotinin 0.25 mg/mL, leupeptin 0.25 mg/mL, pepstatin A 0.25 mg/mL; E-64 0.25 mg/mL).
- Store the cell suspension at -80 °C for the subsequent test purification (see 3.2.2).
- Protein purification from frozen cell suspension in 24-well test-expression blocks.
- Assembly of the binding block (Figure 3).
- Place 3 overlapping layers of parafilm on the top of the 96-deep well block (96-deep well 2.4 mL).
- Place a 96-well filter plate (filter microplate, 96-well, polypropylene with 25 µm ultra high molecular weight polyethylene membrane) on the top of the 96-deep well block.
- Push down the filter plate to secure the tips into the parafilm to seal off the filter plate from the 96-well deep block.
- Transfer 50 µL of pre-equilibrated 50% Ni-NTA resin slurry into each well of the filter plate.
- Test expression procedure
- Place the frozen cell suspension (step 3.1.15) present in 24-well blocks in a water bath at RT for 5-10 min, then shake at 450 rpm for 20 min.
- Centrifuge the 24-well blocks at 3,275 x g for 15 min.
- Using a multichannel pipette, transfer the cleared lysates into a filter plate containing 50 µL of pre-equilibrated 50% Ni-NTA resin slurry (step 3.2.1.4) and seal the filter plate with a 96-well cap mat (96-well cap mat, for use with square well, 2 mL).
NOTE: Use a few rubber bands to keep the binding block together during incubation and centrifugation steps. - Place the secured binding block for 45-60 min into a rotator in a cold room to incubate cleared lysates with Ni-NTA resin.
- After incubation, carefully lift the filter plate and remove the parafilm layer from the surface of the 96-deep well block (step 3.2.1.1).
- Place back the filter plate on top of the 96-deep well block and spin down the secured binding block for 2 min at 235 x g.
- Wash bond Ni-NTA resin 2x with 2 mL of washing buffer comprising 25 mM Tris pH 8.0, 300 mM NaCl, 5% glycerol, and 15 mM imidazole.
- Spin down the block with washing buffer each time for 5 min at 235 x g to ensure complete removal of residual liquid.
- Transfer the filter plate on the top of the 96-well PCR plate containing 10 µL of 4x loading dye.
- Add 40 µL of elution buffer (25 mM Tris pH 8.0, 300 mM NaCl, 5% glycerol, 500 mM imidazole) to each well of the filter plate and incubate for 5 min.
- Spin down the block to elute proteins into the 96-well PCR plate at 235 x g for 10 min.
- Seal the 96-well PCR plate with high temperature resistant tap pad and heat at 98 °C for 3 min.
- Load 15 µL of eluted protein samples in standard Laemmli buffer on 4-20% SDS-PAGE gel next to the protein ladder and run the gel with standard running buffer containing SDS.
- Stain the gel with Coomassie blue and de-stain with water. Analyze the results of test expression to identify the best expressing constructs for large-scale production.
- Assembly of the binding block (Figure 3).
4. Preparations of the baculovirus-infected insect cells (SCBIIC) for protein production
- 4 days before the scheduled production time, split exponentially growing Sf9 cells to a final cell density of 2 x 106 cells/mL into 125 mL / 250 mL / 500 mL Erlenmeyer glass shake flasks with baffles in 50 mL / 100 mL / 200 mL of Insect Serum-Free medium containing 1% (v/v) final antibiotic-antimycotic.
- Add 0.150 mL / 0.300 mL / 0.6 mL of appropriate P2 viruses, incubate infected cells at 165 rpm on an orbital shaker with a one-inch stroke and at a lower temperature of 25 °C to slow down the cell division.
- At 4 days post-infection, check cells under a microscope for SOI and proceed to production if the cell viability as verified with Trypan Blue stain is close to 70-75%.
5. Sf9 cell preparations for large-scale protein production
- 4 days before the scheduled production time, calculate the required volume of Sf9 cells for large-scale protein production.
- Seed 2 L of exponentially growing Sf9 cells in Insect Serum-Free Medium to a cell density of 1 x 106 cells /mL in 2.8 L Fernbach shake flasks.
- Incubate flasks at 27 °C with shaking set at 150 rpm.
NOTE: To prevent bacterial contamination in the Sf9 cell culture, use gentamicin to a final concentration of 10 µg/mL or penicillin/streptomycin to 50 U/mL and 50 µg/mL, respectively.
6. Infection of the Sf9 cells with SCBIIS for the large-scale protein production
- Split 2 L or 4 L of exponentially growing Sf9 cells in Insect Serum-Free Medium to a final cell density of 4 x 106 cells/mL in 2.5 L Tunair shake flasks or 5 L reagent bottles.
- Add 10-12 mL/L of the baculovirus-infected insect cell (SCBIIC) suspension culture.
- Incubate the infected culture of Sf9 cells on a shaker with 145 rpm at the lower temperature of 25 °C (to slow down cell division) for 72-96 h.
- At 72 h post-infection, check cells under a microscope for SOI and assess cell viability.
- Usually, after about 72 h post-infection, the viability of Sf9 cells drops to 70%-75% (measured using Trypan Blue stain). Harvest infected Sf9 cells in a 1 L polypropylene bottle by centrifugation at 900 x g for 15 min at 4 °C.
- Resuspend the cell pellet collected from 1 L of the production cell culture with 20-25 mL of 1x PBS by gently swirling and transfer into 50 mL conical tubes.
- Spin down the cell suspension at 900 x g for 15 min and discard the PBS solution.
- Resuspend the washed cell pellet with 20-25 mL of the suspension buffer (20 mM Tris-HCl pH 8.0, 500 mM NaCl, 5% glycerol, 1x protease inhibitor cocktail) and flash freeze in liquid nitrogen; store at −80 °C until purification.
NOTE: Purification procedures for the PRMTs have been described in detail in the SGC published paper6.
Results
An overview of the BEVS protocol is outlined in Figure 1. Multiple expression constructs of PRMTs, including full-length, domains, and truncated fragments, were generated at the Structural Genomics Consortium (SGC, Toronto) according to in-house strategies with an attempt to increase the success rate for identifying soluble and stable proteins with a relatively high expression level7,9. Interested readers are encouraged to review the SGC's definitions and methodology of designing a "fragment" as the segment of the gene sequence incorporated into an expression clone, "domain" as a PFAM-annotated structural domain, and "construct" as the fragment cloned in an expression vector, all of which have been described in detail in an earlier publication7. Expression constructs of PRMTs presented in this protocol are for the production of the polyhistidine-tagged proteins cloned into the pFBOH-MHL vector, which is a derivative of the pFastBac1 vector. In Figure 4, we present SDS-PAGE analysis of the His-tagged soluble constructs of PRMT1, 2, 4-9 purified from pellets collected after 4 mL of production in Sf9 cells (step 3.1.4). Full-length (FL) PRMT1 and PRMT9 are not presented in this gel, since FL PRMT1 has been produced from E. coli, and FL PRMT9 produced from BEVS has been purified by Flag-tag6. The truncated constructs of PRMT1, FL PRMT4, and all the PRMT8 constructs show a relatively high yield, but protein eluates contain fractions of co-purified contaminants. These constructs require further optimization of the purification protocols. Additional approaches are thus required to improve the purity of these proteins from scale-up productions, such as a reduction in the amount of nickel beads at the stage of the incubation with a clarified lysate; an increase of the imidazole concentrations in the wash buffers; cleavage of the His-tag with TEV protease, followed by application on a Ni-affinity resin; and, additional purification steps such as size-exclusion and ion-exchange chromatography. The constructs of PRMT2 show significantly lower yield compared to other proteins and full-length PRMT2 protein accompanied by a strong contaminant band. Scale-up production and two steps of purifications such as IMAC and size-exclusion confirmed a low expression level for this construct along with the persistent presence of the co-purifying contaminant for the FL protein. Pure proteins have been obtained for the PRMT5 complex produced and purified with its obligate binding partner, MEP50. The truncated construct of PRMT9 has almost two or three-fold lower expression level, close to 1.5 mg/L, as compared to other PRMTs. Nevertheless, the recombinant viral stocks of this construct have been used for scale-up production, diffracting crystals were obtained, and the structure was solved for this protein along with PRMT4, 6, and 7 (Figure 5).
For the scale-up productions, the corresponding P2 viruses were used to infect the suspension culture of Sf9 insect cells. This step generates 50/100/200 mL of baculovirus-infected cells containing infected cells and P3 viruses in the supernatant. For the large-scale protein production, 2 L of Sf9 cells were cultured in each of 2.8 L Fernbach shake flasks at 150 rpm, 27 °C (Figure 6). On the day of production, 2 L of Sf9 cells (cell viability > 97%) in 2.5 L Tunair shake flasks or 4 L in 5 L reagent bottles were diluted to a cell density of 4 x 106/mL. These cells were infected directly with 10-12 mL/L of suspension culture of baculovirus-infected insect cells and incubated at a lowered temperature of 25 °C, at 145 rpm. Infection of the production batch directly with a suspension culture of baculovirus-infected insect cells significantly reduced laborious and time-consuming steps in virus volume amplification, excluding the extra handling of the infected cells, and avoided reduction in titer and virus degradation. SF9 cell culture maintenance and scale-up production have been done in the culture vessels with a high-fill volume to adopt a large-scale protein production in the one batch (Figure 6).
Full-length PRMT 4, 5 (in complex with MEP50), 6, 7, and 9 proteins produced from the Baculovirus mediated production platform have been used for kinetic characterization and inhibitor compound screening at the SGC6. Crystal structures were solved and deposited into the Protein Data Bank (PDB) for the full-length or truncated forms of the proteins PRMT 4, 6, 7, and 9 with various chemical probes and inhibitors. Expression plasmids for these PRMTs were deposited to the Addgene plasmid repository (Addgene is a distributing partner of the SGC, https://www.addgene.org/) and are available to the research community (Figure 5).
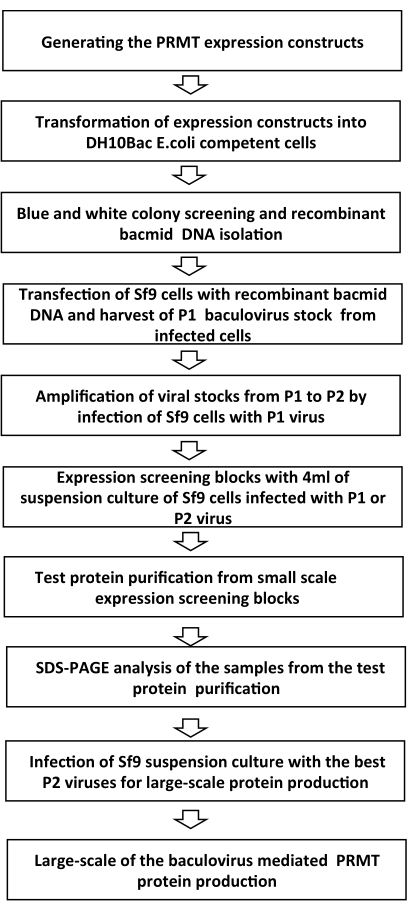
Figure 1: Schematic overview of the steps of the baculovirus expression process Please click here to view a larger version of this figure.
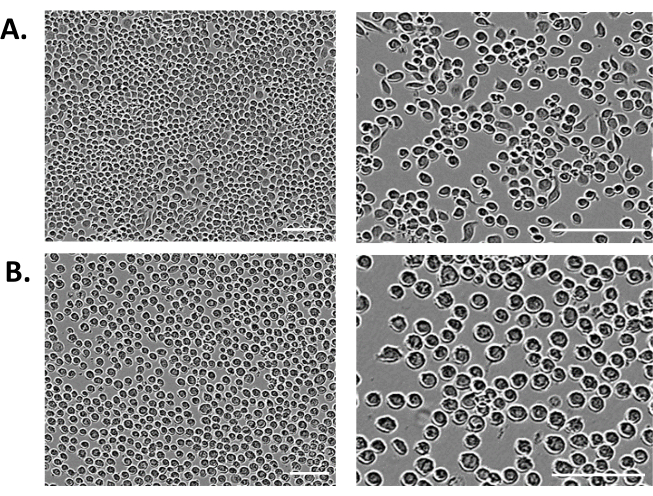
Figure 2: Baculovirus Infected and uninfected Sf9 cells. Signs of infection are structural changes in the insect cells, such as a 25-50% increase in the cell diameter, enlarged cell nuclei, uniformly rounded shape, loss of proliferation, and adherence to the culture dish surface, as well as a decrease in cell viability. White scale bar 200 µm. The signs of infection presented here are the same for the transfected cells using both transfection reagents, JetPrime and X-tremeGene 9. The particular example shown is for the JetPrime transfection reagent. (A) Uninfected Sf9 cells as a control. (B) Baculovirus-infected Sf9 cells. Please click here to view a larger version of this figure.
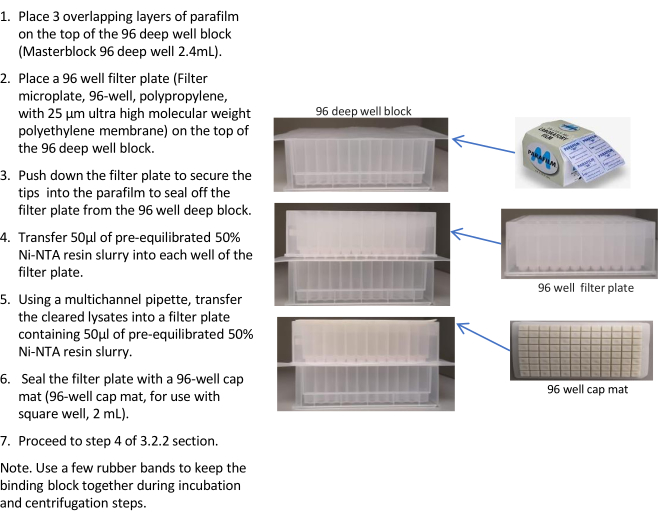
Figure 3: Binding plate assembly for quick purification of test expression proteins. Please see text for details, steps 3.2.1-2 Please click here to view a larger version of this figure.
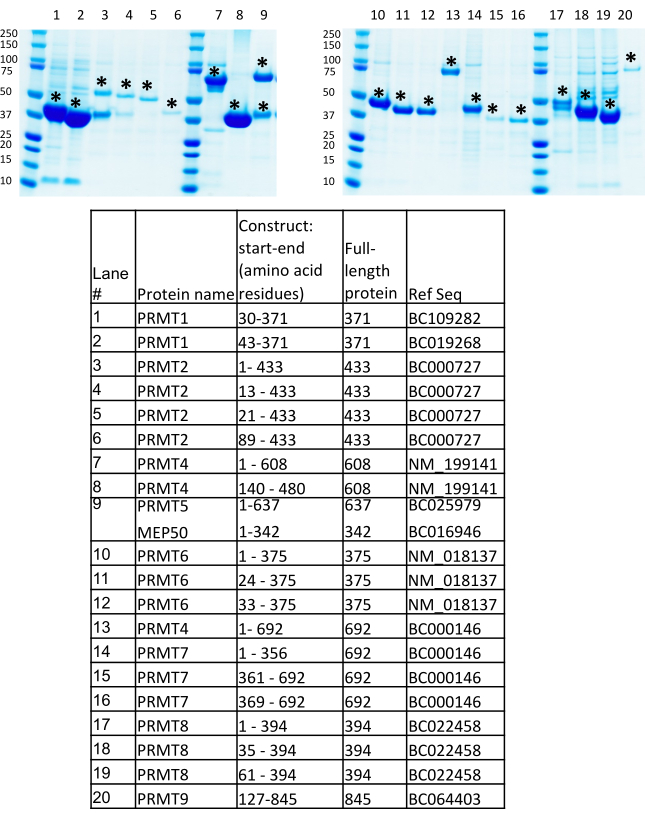
Figure 4: Protein expression screening results. Protein expression screening results of the baculovirus mediated protein production in 4 mL of Sf9 suspension culture infected with corresponding P1 recombinant viruses for different PRMTs and the PRMT5-MEP50 complex. Please click here to view a larger version of this figure.
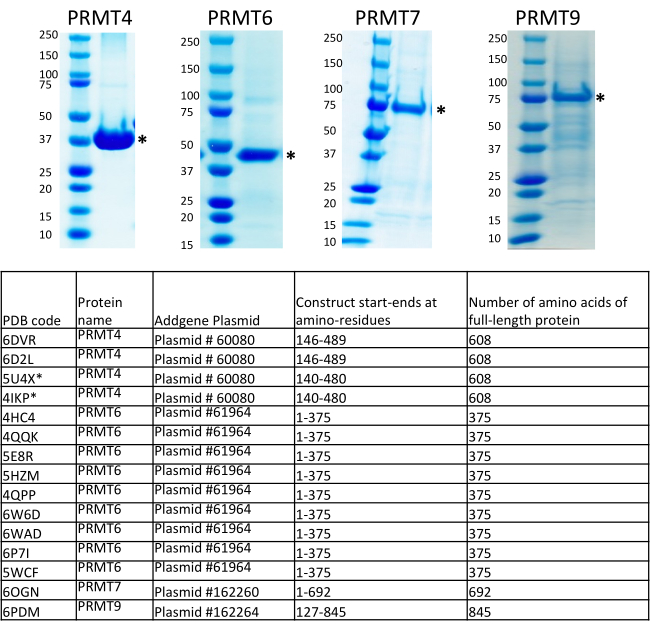
Figure 5: Summary of expression constructs for PRMT4, 6, 7, and 9 used for crystal structure studies at the Structural Genomics Consortium, Toronto (SGC). The crystal structures were solved and deposited into the Protein Data Bank (PDB) for the full length or truncated forms of proteins PRMT 4, 6, 7, and 9 with various chemical probes and inhibitors. Expression plasmids for these PRMTs were deposited to the Addgene plasmid repository and are available to the research community (Addgene is a distributing partner of the SGC, https://www.addgene.org/). Please click here to view a larger version of this figure.

Figure 6: Sf9 insect cell maintenance and protein production in the different culture vessels: (A) 2.8 L Fernbach flask for cell maintenance and protein production. The use of the 72% fill-volume increases the throughput rate by 2.5-fold in one shaking platform. (B) Tunair shake flasks (only 9 flasks out of 10 are presented in this picture) and reagent bottles with an 80% fill-volume drastically increase the shaking platform's production capacity. Please click here to view a larger version of this figure.
Discussion
One of the advantages of BEVS in insect cells centers on the capability of the post-translational modification machinery to enable more complex modifications such as phosphorylation, myristoylation, and glycosylation. Together with the highly efficient folding of mammalian proteins, these modifications facilitate high amounts of modified and folded protein suitable for physiologically relevant downstream experiments16.
Here, we described detailed protocols of the BEVS emphasizing critical elements for successful expression screening of multiple constructs of PRMT proteins and large-scale PRMT protein production in the Baculovirus expression platform: 1) The use of regular, adjustable, and programmable multichannel pipettes to transfer the biological materials between 24- and 96 - well cell culture plates and blocks at the stages of bacmid DNA and virus generation; a collection of the recombinant viruses, amplification of viral volumes of the recombinant viruses and preparation of the protein expression screening blocks. 2) High performance and cost-effective transfection reagents for the generation of recombinant viruses. 3) Suspension culture of baculovirus-infected insect cells (SCBIIC) for large-scale protein production. 4) Utilization of high-fill volume 2.8 L Fernbach shake flasks to maintain Sf9 suspension culture and 2.5 L Tunair shake flasks and 5 L reagent bottles for the large-scale protein production.
Special considerations and rationale for the transformation and transfection steps.
Although a commercial protocol recommends using 100 µL of competent cells for one transformation14, the transformation efficiency of commercial DH10Bac E. coli competent cells is as high as 1 x 108 cfu/ µg DNA, so we use only 4 µL. This is enough for each transformant to obtain isolated white recombinant colonies for the bacmid DNA isolation. Adherent Sf9 cells in the 24-well transfection plate were seeded at a cell density of 2 x 105 /mL in 0.5 mL of Serum-Free Insect Media. This volume is enough to ensure even coverage of the working surface of the well. At the same time, it does not dilute the transfection mix too much, which enhances transfection efficiency. Transfection reagents are non-toxic to the Sf9 cells, and media exchange is not necessary. Instead of a media change, an additional 1.5 mL of media containing 10% (v/v) FBS is added into the transfection plate at 4-5 hours post-transfection time to facilitate cell growth. The transfection efficiency of both transfection reagents is high. Still, with X-tremeGene 9, the signs of infection in the transfected cells (Figure 2) appear 10-12 h earlier than with the JetPrime reagent, so we choose between these reagents depending on the working schedule of the next steps in the protocols, which provides some flexibility in the overall process.
Protein test expression screening can be set up with P2 viruses if the amount of the initial recombinant viruses, collected from the transfection plate and labeled as P1, is a limiting factor to use for the protein expression screening.
Considerations when moving from small to large culture volumes.
Historically, it was believed that optimal cell growth requires a high air space in the suspension culture of Sf9 cell maintenance and scale-up productions. However, in 2014, it was reported that high air space in culture vessels is less critical than previously thought17. A culture vessel set up using appropriately adjusted shaking speed to the orbital throw of the shaking platform will provide sufficient oxygen transfer even in the high-fill volume suspension culture by creating and maintaining small air bubbles for a longer time. With this approach, commercially available insect cells can be cultured at a higher shaking speed within a normal range of the cells' doubling time without sacrificing high cell viability.
Thus, 6 years ago, we started to increase the suspension culture volume in a shaking flask during cell maintenance and introduced a different type of culture vessel for protein production while adjusting and monitoring shaking conditions (Figure 6). To establish optimal conditions in these culture vessels, we monitored the Sf9 cell culture parameters such as cell doubling time along with cell viability, size and shape, state of aggregation, and infectability of cells.
For example, for Sf9 cell maintenance, in the 2.8 L Fernbach shake flasks, we culture 2 L instead of 0.8 L of the Sf9 suspension cells shaking at 150 rpm at 27 °C and cell viability most of the time is close to 99%, with evenly shaped healthy dividing cells. For scale-up production, we infect 4 L of cells in 5 L reagent bottles shaking at high speed as 145 rpm at a lowered temperature of 25 °C. The most commonly used incubators with a built-in shaking platform can hold 6 x 2.8 L shake flasks or 6 x 5 L reagent bottles, or 10 x 2.5 L Tunair shake flasks. Thus, the capacity of the one shaking platform, if we fill Fernbach shake flasks and reagent bottles to 1/3 versus to the high-fill volume of the vessels is 4.8 L versus 12 L using 2.8 L Fernbach shake flasks and 10 L versus 24 L using 5 L reagent bottles (Figure 6). Suspension cell culture maintenance and scale-up production in the culture vessels with a high-fill volume have helped us overcome limitations of the production volumes and adopt a large-scale platform. Thus, this is highly useful for labs with no access to bioreactors and/or limited space in the production pipelines.
This protocol could be readily adapted for the production and purification of protein constructs with different affinity tags by utilizing appropriate resins and modifying purification buffers as has been described in the SGC published paper6 for the Flag-tagged full-length proteins of PRMT4, 7, 9, and the His-tagged PRMT5-MEP50 complex and PRMT6. Although we describe a BEVS protocol for the PRMT family of proteins, the same approach can be applied to any other protein family.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
The authors wish to thank Dalia Barsyte-Lovejoy for taking the time to provide valuable feedback and critical comments on the manuscript and all our SGC colleagues who worked with the PRMT protein family expressed from the Baculovirus Expression Vector System.
The SGC is a registered charity (number 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], the EU and EFPIA through the Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA (aka EMD in Canada and US), Pfizer, Takeda and the Wellcome Trust [106169/ZZ14/Z].
Materials
| Name | Company | Catalog Number | Comments |
| 2.8L Nalgene Fernbach Culture Flask, Polycarbonate, | Nalgene | 29171-854 | For large scale maintenance of suspension culture of Sf9 cells |
| 24-Well Blocks RB | Qiagen | 19583 | For incubation of 4ml of suspension Sf9 cells for protein expression screening |
| 4-20 Criterion TGX Gel 26W 15 ul | Biorad | 5671095 | For SDS-PAGe analysis of the purified proteins |
| 50ml Reagent Reservoir | Celltreat Scientific Products | 229290 | Reservoir used for diluted transfection reagent and Sf9 cells suspension |
| 96-well cap mat, for use with square well, 2 mL | Greiner Bio-One | 381080 | Used to cover 96 well block |
| 96 well PCR plate | Eppendorf | 30129300 | |
| Bacto agar | BD | 214010 | For LB-agar selection paltes |
| Airpore Tape Sheets | Qiagen | 19571 | To cover 24 well blocks for protein expression screening |
| Allega X-15R Centrifuge | Beckman Coulter | 392932 | |
| Antibiotic Antimycotic (100x) | Gibco | 15240112 | |
| Bacmid DNA | in-house | non-catalog item | Bacmid DNA for baculovirus production |
| Bluo-Gal, 1g | Thermo Fisher | 15519028 | |
| Beckman JLA 8.1000 | |||
| Cell Culture Plates, 24-Well, with lid, flat bottom, sterile | Eppendorf | 30722116 | Tissue culture treated plate |
| Cell Resuspension Solution 0.5 L | Millipore Sigma | LSKCRS500 | For Bacmid DNA extraction |
| Cell Lysis Solution 0.5 L | Millipore Sigma | LSKCLS500 | For Bacmid DNA extraction |
| CELLSTAR Tissue Culture Plates, 96 well | Greiner Bio-One | 655180 | For transfection mix |
| ClipTip 1250, filter reload, sterile | ThermoFisher Scientific | 94420818 | Tips for programmable and adjustable multichannel pipette |
| dNTP Mix (25 mM each) | Thermo Fisher Scientific | R1121 | |
| E1-ClipTip Electronic Adjustable Tip Spacing Multichannel Equalizer Pipette, 15 to 1250 μL | ThermoFisher Scientific | 4672090BT | Programmable and adjustable multichannel pipette |
| E4 XLS adjustable spacer 6-channel pipette, 20-300 μL | Ranin | LTS EA6-300XLS | Ranin adjustable multichannel pipette |
| Filter microplate, 96-well, polypropylene, with 25 µm ultra high molecular weight polyethylene membrane | Agilent | 201005-100 | For protein purification in expression screening |
| Full-Baffle Flask Kit Tunair, 2.5L | IBI Scientific | SS-6003C | For large scale protein production in suspension culture of SF9 cells |
| Gentamicin 10x10ml | BioShop | 15750078 | |
| Heat Inactivated Fetal Bovine Serum | Wisent Biocenter | 080-450 | |
| I-Max Insect Media W/ L-Glutamine, 1 L | Wisent Biocenter | 301-045-LL | Serum free insect cells growth medium |
| InstantBlue, Ultrafast Protein Stain | Expedeon Protein Solutions | ISB1L-1L | For protein gel (SDS-PAGE) staining |
| Iptg, Ultra Pure, Dioxane Free, Min 99.5% | BioShop | IPT001.100 | |
| JetPRIME Transfection Reagent Provided with jetPRIME buffer | POLYPLUS TRANSFECTION Inc | 114-01 | For Sf9 cells transfection to generate baculovirus |
| Kanamycin Monosulfate | BioShop | KAN201.100 | |
| Lb Broth (Lennox), Powder Microbial Gro& | Sigma | L3022-1KG | |
| Masterblock 96 deep well 2.4mL | Greiner Bio-One | 780285-FD | Used in the transformation and expression screening procedures |
| Max Efficiency DH10Bac Competent Cells , 0.5ml | Thermo Fisher | 10361012 | Competent cells for bacmid DNA generation |
| mLINE 12-Channel Pipette, adjustable 30 - 300 uL | Sartorius | Sartorius 725240 | 12 channel pipette |
| Neutralization Solution, 0.5 L | Millipore Sigma | LSKNS0500 | For bacmid DNA extraction |
| New Brunswick Innova 44R, 120V, orbit 2.5 cm (1 in) | Eppendorf | M1282-0004 | Shaker incubator for incubaion of suspension of Sf9 cells |
| Ni-NTA Agarose | Qiagen | 30250 | For protein purification |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | LS15140122 | |
| PYREX Delong Shaker Erlenmeyer Flask with Baffles, Corning 500ml | Pyrex | 4444-500 | For suspension culture of Sf9 cells |
| PYREX Delong Shaker Erlenmeyer Flask with Baffles, Corning 125ml | Pyrex | 4444-125 | For suspension culture of Sf9 cells |
| PYREX Delong Shaker Erlenmeyer Flask with Baffles, Corning 250ml | Pyrex | 4444-250 | For suspension culture of Sf9 cells |
| RedSafe Nucleic Acid Staining Solution | Froggabio | 21141 | |
| RNase A, 0.9 mL | Millipore Sigma | LSKPMRN30 | For suspension buffer for bacmid DNA extraction |
| Roll & Grow Spherical Glass Plating Beads | MP Biomedicals | 115000550 | For spread of bacterial cells across the surface of an agar plate. |
| RT-LTS-A-300μL-768/8 (tips) | Ranin | 30389253 | Tips for ranin multichannel pipette |
| S.O.C. Medium | Thermo Fisher Scientific | 15544034 | |
| Serum, Cell Culture, Fetal Bovine Serum (Fbs), Hyclone, Characterized Canadian | cytivalifesciences | SH3039602 | Addition to the serum free medim for the transfected cells |
| Sf9 cells | Thermo Fisher | 12659017 | Insect cells |
| Sfx-Insect Cell Culture Media | Cytiva (Formerly GE Healthcare Life Sciences) | SH3027802 | Serum free insect cells growth medium |
| Tape Pad | Qiagen | 19570 | Tape Pad |
| Taq DNA Polymerase with ThermoPol Buffer - 2,000 units | New England Biolabs | M0267L | |
| Tetracycline Hcl | BioShop | TET701.10 | |
| Trypan Blue 0.4% Solution | Gibco | 15250061 | For assessment of cell viability |
| VITLAB Reagent Bottles, PP with Screw Caps, PP, BrandTech, 5L | VITLAB | V100889 | For large scale protein production in suspension culture of Sf9 cells |
| VWR Digital Mini Incubator | VWR | 10055-006 | Incubator for adherent Sf9 cells |
| VWR Incubating Microplate Shaker | VWR | 97043-606 | Incubator for suspension culture of Sf9 cells in 24 well blocks |
| VWR Petri Dishes | VWR | CA73370-037 | |
| X-tremeGene 9 DNA Transfection Reagent 1.0 M | Roche | 6365787001 | For Sf9 cells transfection to generate recombinant baculovirus |
References
- Guccione, E., Richard, S. The regulation, functions and relevance of arginine methylation. Nature Reviews in Molecular Cell Biology. 20 (10), 642-657 (2019).
- Thandapani, P., O'Connor, T. R., Bailey, T. L., Richard, S. Defining the RGG/RG motif. Molecular Cell. 50 (5), 613-623 (2013).
- Lorton, B. M., Shechter, D. Cellular consequences of arginine methylation. Cell and Molecular Life Sciences. 76 (15), 2933-2956 (2019).
- Bedford, M. T., Clarke, S. G. Protein arginine methylation in mammals: Who, what, and why. Molecular Cell. 33 (1), 1-13 (2008).
- Frankel, A., Brown, J. I. Evaluation of kinetic data: What the numbers tell us about PRMTs. Biochimica Biophysica Acta Proteins Proteomics. 1867 (3), 306-316 (2018).
- Li, A. S. M., Li, F., Eram, M. S., Bolotokova, A., Dela Seña, C. C., Vedadi, M. Chemical probes for protein arginine methyltransferases. Methods. 175, 30-43 (2020).
- Savitsky, P., et al. High-throughput production of human proteins for crystallization: The SGC experience. Journal of Structural Biology. 172, 3-13 (2010).
- Gileadi, O., et al. Expressing the human proteome for affinity proteomics: Optimizing expression of soluble protein domains and in vivo biotinylation. New Biotechnology. 29 (5), 515-525 (2012).
- Graslund, S., et al. Protein production and purification. Nature Methods. 5, 135 (2008).
- Kost, T. A., Condreay, J. P., Jarvis, D. L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nature Biotechnology. 23, 567-575 (2005).
- Jarvis, D. L. Baculovirus-insect cell expression systems. Methods in Enzymology. 463, 191-222 (2009).
- Shrestha, B., et al. Baculovirus expression vector system: an emerging host for high-throughput eukaryotic protein expression. Methods in Molecular Biology. 439, 269-289 (2008).
- Smith, G. E., Summers, M. D., Fraser, M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Molecular Cell Biology. 3, 2156-2165 (1983).
- Invitrogen Life Technologies. . Invitrogen, Bac-to-Bac Baculovirus expression system. , (2010).
- Luckow, V. A., Lee, S. C., Barry, G. F., Olins, P. O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. Journal of Virology. 67, 4566-4579 (1993).
- Irons, S. L., Chambers, A. C., Lissina, O., King, L. A., Possee, R. D. Protein Production Using the Baculovirus Expression System. Current Protocol in Protein Sciences. 91, 1-22 (2018).
- Rieffel, S., et al. Insect cell culture in reagent bottles. MethodsX. 1, 155-161 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved