Method Article
A Human Corneal Organ Culture Model of Descemet's Stripping Only with Accelerated Healing Stimulated by Engineered Fibroblast Growth Factor 1
In This Article
Summary
Descemet's Stripping Only is an experimental procedure wherein patients with central corneal guttae resulting from Fuchs Endothelial Corneal Dystrophy have Descemet's membrane stripped for peripheral cells to regenerate the endothelial layer. We present novel methodology simulating DSO in dystrophic human corneas ex vivo with accelerated healing stimulated by eFGF1 (NM141).
Abstract
Fuchs Endothelial Corneal Dystrophy (FECD) results from dysfunctional corneal endothelial cells (CECs) and is currently treated by transplantation of the whole cornea or Descemet's membrane. Recent developments in ocular surgery have established Descemet's Stripping Only (DSO), a surgical technique in which a central circle of guttae-dense Descemet's membrane is removed to allow for the migration of CECs onto the smooth stroma, restoring function and vision to the cornea. While this potential treatment option is of high interest in the field of ophthalmic research, no successful ex vivo models of DSO have been established and clinical data is limited. This work presents a novel wound-healing model simulating DSO in human donor corneas. Using this approach to evaluate the efficacy of the human engineered FGF1 (NM141), we found that treatment accelerated healing via stimulation of migration and proliferation of CECs. This finding was confirmed in 11 pairs of human corneas with signs of dystrophy reported by the eye banks in order to verify that these results can be replicated in patients with Fuchs' Dystrophy, as the target population of the DSO procedure.
Introduction
Fuchs Endothelial Corneal Dystrophy (FECD) is a disease characterized by lost pump function in corneal endothelial cells (CECs) and the excessive buildup of collagen and other extracellular matrix proteins on the surface of Descemet's membrane, forming corneal guttae1. The only known treatment for FECD is endothelial keratoplasty in varying forms, all of which come with risk of rejection and endothelial cell loss2. While advancements in ophthalmic surgery have enabled these procedures to become less invasive over time, any form of transplantation comes with the risk of rejection and possibility of lifetime steroid use, a treatment with its own concomitant adverse events. Moreover, the global donor tissue shortage is such that only one donor cornea is available for every 70 patients in need3. Given these challenges, researchers and clinicians are exploring surgical methods that avoid the need for donor tissue altogether. One of these experimental techniques is Descemet's Stripping Only (DSO) or Descemetorhexis without Endothelial Keratoplasty (DWEK), in which FECD patients with guttae localized to the center of the cornea have a central 4 mm circle of Descemet's membrane stripped without graft placement. The removal of guttae encourages healthy peripheral cells to migrate inward and reform the endothelial monolayer, eventually reversing stromal edema and improving vision. The concept was originally described in a series of case studies in which patients underwent surgery that was complicated by detachment of Descemet's membrane, but CEC repopulation still occurred4,5,6,7. Though there are many advantages to this method, the healing process is lengthy and inconsistent, as some patients require rescue transplantation if no healing is seen in the months following surgery8. For these reasons, a drug that stimulates faster migration and proliferation of CECs may be beneficial in the recovery process of FECD patients that have undergone DSO.
Several recent studies have evaluated ROCK inhibitors as a supplemental treatment for patients undergoing DSO, and found that treated patients recovered faster and had higher central endothelial cell densities (ECD) than those in the DSO only group9,10,11. However, due to small sample sizes and differences between dosing regimens, more data is needed to better understand the efficacy of ROCK inhibitors in this setting.
Fibroblast Growth Factors have also been shown to stimulate regeneration of the corneal endothelium both in vitro with bovine CECs, and in vivo in feline corneas12,13. eFGF1 (NM141) is an engineered version of FGF-1 containing several amino acid substitutions to stabilize the molecule, as opposed to the native FGF-1, which has a much shorter half-life14,15. We have previously demonstrated the ability of eFGF1 (NM141) to stimulate proliferation of CECs ex vivo in quartered human corneas16. This study sought to improve upon that work by establishing the first successful ex vivo model of DSO in both normal and dystrophic corneas to determine whether adjunctive treatments such as eFGF1 (NM141) accelerate healing in this application.
Protocol
This work used existing specimens without identification of subjects and is exempt from IRB approval under 45 CFR 46.101(b)(4).
Human donor corneas were obtained from various eye banks across the US (see Table of Materials). Corneas were individually judged dystrophic by the eye banks based on findings such as guttae, pleomorphism, polymegathism, and/or low ECD upon specular evaluation. Figure 1 presents Trypan Blue staining in the cornea during wound creation, immediately following stripping, and after 2 weeks in culture.
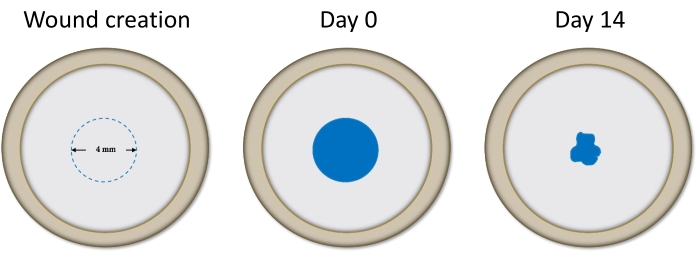
Figure 1: Diagram representing cornea during wound creation, immediately post-stripping, and after 14 days in culture as visualized by Trypan Blue. The reduction in stained area between these timepoints was measured to determine the level of healing. Please click here to view a larger version of this figure.
1. Wound creation
- Using sterile forceps, remove the cornea from media and rinse in 1x PBS to remove residual media and cell debris. After rinsing, place the cornea endothelial side up on the lid of a Petri dish.
- Pipette 30 µL of Trypan Blue into a welled dish. Dip a new 4 mm biopsy punch in Trypan Blue and tap off any excess.
- Using both hands, position the punch above the center of the cornea and lower it straight down onto the endothelial surface, applying minimal pressure.
- Without changing the position or pressure on the cornea, shift the punch to one hand and reach for forceps with the newly freed hand. Use the forceps to hold the cornea in place while gently twisting the biopsy punch about 90° back and forth several times.
- Lift the punch straight up from the cornea and set aside.
- Rinse once more in 1x PBS to remove excess Trypan Blue.
2. Descemet's stripping
- Transfer cornea on a Petri dish lid to a dissecting scope.
NOTE: Do not leave cornea dry longer than 5 min. If more time is needed to complete the process, rinse again in 1x PBS to rehydrate the endothelium. - Holding the cornea in place with curved forceps, score Descemet's membrane by lightly dragging the tip of a sharp 30 G needle along the ring of Trypan Blue left by the biopsy punch. Use minimal pressure to avoid disrupting the underlying stroma.
- With a Sinskey hook, use gentle scooping motions to lift and peel back Descemet's membrane around the wound edge, working toward the center of the lesion.
NOTE: Descemet's membrane should come up with very little resistance. If difficulty is experienced, one is likely pulling up stroma. If this happens, start again, lifting from a new point along the wound edge. - Once the majority of Descemet's membrane has been separated from the stroma, use Gorovoy forceps to remove the membrane and set aside.
- Examine the stripped area for any remaining pieces of membrane and remove with Gorovoy forceps.
3. Trypan Blue staining
NOTE: Following Descemet's stripping, stain the cornea with Trypan Blue to visualize the wound area.
- Return the cornea on a Petri dish lid to the biosafety cabinet and rinse in 1x PBS containing 0.01% (w/v) CaCl2 and MgCl2 to remove cell debris and facilitate tight adherence of remaining CECs to the intact Descemet's membrane.
- Place cornea in a welled dish and pipette 30 µL of Trypan Blue onto the endothelial layer for 30 s.
- Use forceps to gently rock the cornea to ensure that the entire endothelial surface is covered.
- Rinse off excess Trypan Blue in 1x PBS with 0.01% (w/v) CaCl2 and MgCl2 and image the stained cornea under a dissecting microscope for the Day 0 timepoint.
- Be sure cornea is balanced such that light is evenly transmitted throughout and positioning is replicable across timepoints.
NOTE: Forceps can be used to hold cornea in place while imaging if needed.
- Be sure cornea is balanced such that light is evenly transmitted throughout and positioning is replicable across timepoints.
4. Culture period
- Culture corneas in a six-well plate containing low-serum media consisting of OptiMEM; 1x insulin, transferrin, and selenium; 1x antibiotic/antimycotic; 0.02 mg/mL CaCl2; 0.2 mg/mL ascorbic acid; and 0.8% heat inactivated fetal bovine serum. Culture the left cornea in 8 mL of low-serum media alone, and the right cornea in 8 mL of low-serum media supplemented with 100 ng/mL eFGF1 (NM141).
- Incubate at 37 °C with 6% CO2 for 14 days with daily media changes.
- Repeat the Trypan Blue staining procedure on Days 3, 6, 9, 12, and 14, and image each cornea immediately after staining. Be sure to keep camera settings consistent across all timepoints.
- On Day 12, add 10 µM EdU to both control and eFGF1 (NM141)-supplemented media and incubate for 48 h to label proliferating cells. Renew media with EdU added again on Day 13.
- On Day 14, 48 h after the addition of EdU, Trypan stain and image corneas as usual, except instead of 1x PBS with CaCl2 and MgCl2, use plain 1x PBS for rinses to prevent the calcium from interacting with the Alizarin Red stain.
5. Alizarin Red staining
- On Day 14, prepare 5% Alizarin Red in 0.9% saline solution.
NOTE: Alizarin Red will not dissolve completely in saline solution. Upon combining, vortex solution and rock for at least 1 h. Vortex once more and filter before use to remove undissolved particles. - Once Trypan staining and imaging have been completed, transfer corneas to a welled dish and add 200 µL Alizarin Red to the endothelial surface of each cornea.
- Stain for 2 min, then rinse each cornea in a Petri dish of 1x PBS to remove excess Alizarin Red before placing in a six-well plate pre-filled with 8 mL of 1x PBS for two 5 min washes.
- After the second wash, image the stripped area of each cornea under a dissecting microscope.
NOTE: Unlike with Trypan imaging, corneas can be left in 1x PBS to image Alizarin staining to prevent them from drying out during the process.
6. Fixation and permeabilization
- Once Alizarin images have been captured, transfer corneas to a 12-well plate and wash in 4 mL of 1x PBS containing 0.05% Tween-20 (PBS-T) for 30 min to remove any particles from tissue prior to fixation.
- Fix corneas in 4 mL of 4% PFA for 30 min at room temperature.
- Permeabilize cells for 5 min in 4 mL of 1x PBS containing 1% Triton X-100, then wash three times in 4 mL of plain 1x PBS for 30 min each.
7. Immunohistochemistry
- Block corneas for 1 h at 37°C in 4 mL of 1x PBS containing 2% bovine serum albumin and 2% goat serum.
- Incubate in 1 mL of blocking solution containing 2.5 µg/mL primary antibody mouse anti-ZO-1 for 1 h at 37°C, then wash three times in 4 mL of 1x PBS for 30 min each.
NOTE: The plate can be tilted during antibody staining to minimize the volume of reagents needed. Just ensure that the entire cornea is completely submerged in antibody solution. - Perform the EdU Click-iT reaction using a cocktail containing 0.88 mg/mL ascorbic acid, 0.26 mg/mL CuSO4, and 2.5 µM Alexa Fluor 488 azide.
- Prepare reaction components
- Add 500 µL of 1x PBS to 88 mg of ascorbic acid and vortex until dissolved.
- Add 840 µL of deionized water to 44 mg of CuSO4 and vortex until dissolved.
- Prepare cocktail by adding the following reagents to 6 mL of 1x PBS in this order, inverting tube to mix after each addition: 30 µL of ascorbic acid solution, 30 µL of CuSO4 solution, and then 15 µL of Alexa Fluor 488
NOTE: Once prepared, this solution is light sensitive. For the remainder of the protocol, corneas should be protected from light whenever possible. - Add 1 mL of EdU reaction cocktail to each cornea and react for 1 h at room temperature.
- Prepare reaction components
- Add 4 mL of 10 µg/mL Hoechst 33342 to each cornea and react for 2 min at room temperature, then wash three times in 1x PBS-T for 30 min each.
- Incubate in 1 mL of blocking solution containing 2 µg/mL Alexa Fluor 555 labeled goat anti-mouse IgG for 1 h at 37°C, then wash three times in 1x PBS-T for 30 min each.
- Store corneas at 4 °C in 4 mL of 1x PBS with 1% anti/anti, 0.1% ciprofloxacin, and 0.1% Amphotericin B to prevent microbial growth.
- When performing confocal imaging, mount whole corneas in 200 µL of mounting medium on glass slides with coverslip taped down to flatten.
8. Trypan Image Analysis
- Perform all image analysis using NIH's open-source software ImageJ. Open an image using File > Open and click Image > Adjust > Color threshold.
- Use the "Hue" sliders to encompass only the blue range and adjust the upper "Brightness" slider all the way to the left to include more of the stained area.
NOTE: If this action causes parts of the image that are not Trypan stained to be included, the "Brightness" slider can be returned to its original level. - Slowly adjust the upper "Saturation" slider until the thresholding accurately outlines the stained area.
NOTE: It is helpful to have a copy of the original image open to refer to during this process. - Click the Select button within the color threshold menu, then use the selection brush tool to de-select areas outside of the wound that were picked up.
- Click Analyze > Measure to report the stained area in pixels.
- De-select all using Edit > Selection > Select None and use the oval selection tool to fit the limbus as closely as possible, then click Analyze > Measure to report the area of the cornea in pixels.
NOTE: Brightness can be turned up temporarily to aid in visualization of the limbus if this part of the image is too dark. - Record area values and divide the stained area by the area of the whole cornea, then multiply by 100 to calculate the percent stained.
- Repeat this process two more times for each image, then take the average of the three measurements.
9. Statistical Analysis
- Once all images have been analyzed, divide the average percent stained on Day 14 by the percent stained on Day 0 to determine the residual unhealed area. Subtract this amount from 100% to calculate the percentage of the stripped area healed over 14 days.
- Use a paired t-test to compare percent healed values between control and treatment groups.
Results
This experiment was initially performed in 10 pairs of normal research corneas, as they are most readily available. Once the method was shown to be successful, the study was replicated in 11 pairs of dystrophic corneas-labeled as such by eye banks based on specular evaluation-to study the effects of eFGF1 (NM141) in corneas representative of the FECD patient population. For greater clinical relevance, the figures included in the present work represent data collected from dystrophic corneas unless otherwise noted.
After simulating the surgical outcome of DSO, Trypan Blue staining was performed and repeated throughout the 14 day culture period, and the reduction in positive staining quantified to evaluate wound healing (Figure 2). Alizarin Red staining was also performed on Day 14 to delineate cell borders. Dark red areas with no clear pattern visible (referred to hereafter as negative staining) appeared consistently within the unhealed section of the lesion and in other areas of the cornea where cells were presumed to be damaged or missing. Areas that stained positive for Trypan Blue on Day 14 corresponded well with negative staining by Alizarin Red (Figure 2). All dystrophic corneas treated with eFGF1 (NM141) showed greater healing on Day 14 compared to the untreated mate. On average, treated corneas demonstrated 91% healing as opposed to 38% in control corneas, and this difference was statistically significant (p < 0.001) (Figure 3A). This is comparable to results obtained from 20 normal corneas, where controls showed an average of 32% healing and treated corneas reached 81%, which again was statistically significant (p < 0.001) (Supplemental Figure 1). The percent healed was compared between normal and dystrophic corneas using two sample t-tests assuming equal variance, and no significant difference was seen in either the control or treatment groups (data not shown).
Donor information provided by eye banks (Table 1) for both normal and dystrophic corneas was analyzed to determine whether characteristics including age, days stored in Optisol, death to collection interval, cell density, and sex correlate with healing. Pearson's correlation coefficient (r) was used to investigate all factors except sex, which was evaluated using an unpaired t-test to compare healing between males and females. The absolute value of r was below 0.25 in all cases, indicating that any correlations between healing and age, days stored in Optisol, death to collection interval, or cell density were considered negligible. The difference in healing between males and females was not statistically significant (p = 0.53).
Similar to the normal data set, 10 of the 22 dystrophic corneas presented with notable Trypan staining outside of the stripped area connected to the stained area within the lesion in at least one timepoint (usually Day 3)-an observation we termed peripheral staining (Figure 4A). Of those 10, only two were control corneas whose treated counterparts did not exhibit the same effect. The remaining eight were all matched pairs and presented with similar severity between corneas from the same individual. Although staining patterns were comparable between normal and dystrophic corneas, the frequency of peripheral staining was higher in the dystrophic data set with 45% of corneas positive, compared to 25% in the normal group. Figure 4A shows a pair that was considered positive for peripheral staining, as the stained area outside of the lesion met the wound edge in the Day 6 images and gradually receded. In the corresponding Alizarin Red images, cells in areas with peripheral Trypan staining appeared enlarged and abnormally shaped, much like the cells that migrated into the stripped area, and negative staining correlated with the areas where Trypan Blue was present on Day 14. Despite the large portion of the cornea affected throughout the culture period, partial or complete clearing of the stained periphery occurred in all 10 corneas. Additionally, the final percent healed at Day 14 was within the normal range, respective to treatment group, for all but one cornea. The outlier (0811L, pictured in Figure 4A) returned a negative percent healed value due to the remnants of peripheral staining that still connected to the lesion area at Day 14 (Figure 3A and Figure 4A). A second staining pattern, referred to as far peripheral staining, was captured in the Alizarin Red images from Figure 4B, but was also apparent in Trypan Blue images throughout the culture period in many corneas (Figure 4B). This observation is characterized by a ring of dark staining around the limbus, indicating compromised endothelium. Despite the term, this pattern was so common in both normal and dystrophic corneas that they were not counted as positive for peripheral staining. In these images, the control cornea showed more vibrant and extensive staining on Day 14, despite both corneas possessing similar severity and pattern of staining early in the culture period. Also noted in the treated cornea was a seemingly larger number of cells, which appeared more compact and hexagonal compared to control, though these observations were not quantitatively measured. Due to the curvature in the periphery, only Trypan staining within and immediately surrounding the stripped area was included in quantitative analysis. When percent-stained values were averaged across all dystrophic corneas, the emergence of peripheral staining resulted in an initial increase from that of Day 0, as opposed to the steady decrease seen in normal corneas where peripheral staining was less prevalent (Figure 3B).
Immunohistochemistry visualized by confocal imaging reveals semi-organized expression of ZO-1, a functional marker of CEC tight junctions, in and around the lesion edge (Figure 5), a pattern similarly evidenced by the Alizarin Red staining (Figure 2 and Figure 4). Pleomorphism and polymegathism are visible by ZO-1 in most dystrophic corneas; however, this observation can be attributed to their diseased state and was noted by the eye bank upon specular examination of the corneas prior to culturing. Disorganized ZO-1 expression is observed within the lesion area of treated corneas where cells had migrated inward, also consistent with the Alizarin Red patterns. EdU-incorporating cells can be seen around the border of the lesion and inside of the lesioned area in control and treated corneas (Figure 5). The pattern of migrated CECs also reflects the Trypan Blue results, where in control corneas most cells were found within two fields of the lesion edge, while CECs in treated corneas can be seen throughout the stripped area (Figure 2).
EdU labeling was performed as a qualitative measure in this study to confirm that proliferation contributed to healing. However, quantification of cells incorporating EdU could not be performed systematically due to corneal swelling preventing consistent, replicable images from being captured in and around the lesion. As a surrogate, quantitative analysis performed in a study conducted prior to establishment of the DSO model has been included to demonstrate the effects of eFGF-1 (NM141) on proliferation (Figure 6). Normal human donor corneas were cut into quarters using a scalpel and cultured in the presence of EdU, with or without eFGF1 (NM141) for 48 h as described previously (Eveleth, 2020)16. Imaging and subsequent analysis of Hoechst 33342 and EdU-labeled cells was performed separately in the undisturbed mid zone of the quarters and in the edge zone, within three fields from where the cornea was cut. In the mid zone, the percentage of cells incorporating EdU was low and highly variable across the 10 corneas analyzed. On average, quarters treated with eFGF1 (NM141) had higher levels of EdU incorporation in the mid zone (4.1% ± 7.9%) compared to controls (1.8% ± 2.9%), though this difference was not statistically significant (p = 0.239). At the wound edge, treated quarters stimulated with eFGF1 (NM141) showed significantly higher rates of EdU incorporation when averaged (18.1% ± 11.5%) compared to control quarters (10% ± 9.6%, p = 0.012).
Table 1: Donor information for 22 dystrophic and 20 normal corneas used in this study, as well as 10 normal corneas used previously for EdU quantitation. Please click here to download this Table.
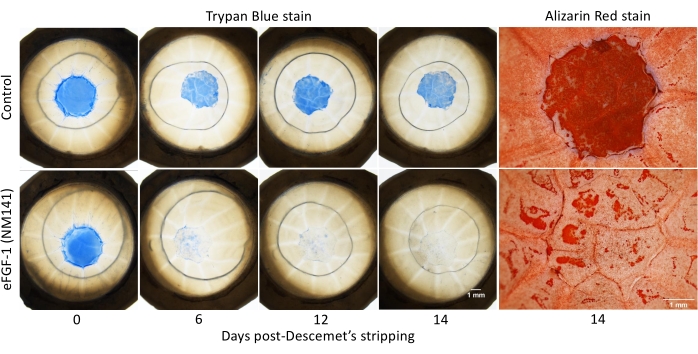
Figure 2: Vital dye staining of corneas post-Descemet's stripping. Dystrophic human corneas were stripped of the central 4 mm of Descemet's membrane and incubated with or without eFGF1 (NM141) (100 ng/mL) for 14 days. Lesions were visualized by Trypan Blue staining on days 0, 3, 6, 9, 12, and 14. CEC borders were visualized by Alizarin Red staining on day 14. Please click here to view a larger version of this figure.
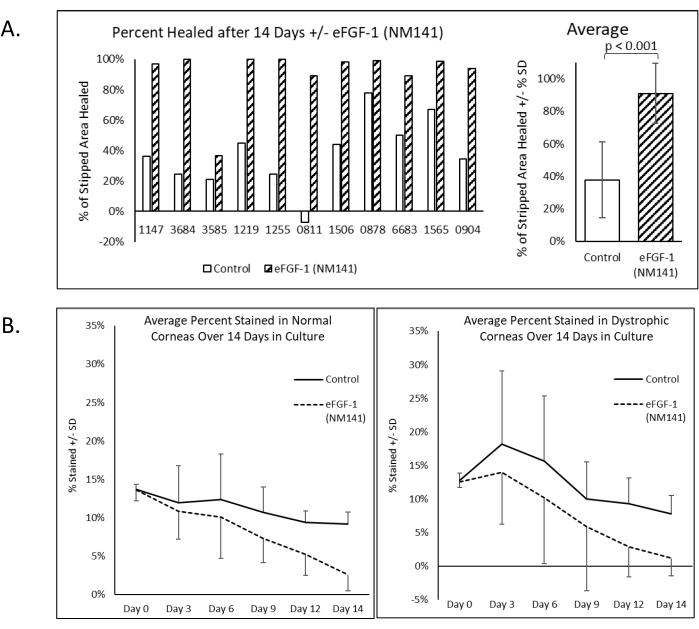
Figure 3: Dystrophic human corneas show significantly greater healing from DSO when cultured with eFGF1 (NM141). (A) Percent healing was determined by measuring the stained area at Day 14 using ImageJ and comparing that to the percent stained at Day 0. (B) The average percent stained graphed over time shows a consistent decrease in normal corneas while peaking at Day 3 in dystrophic corneas due to the higher prevalence of peripheral staining. Please click here to view a larger version of this figure.
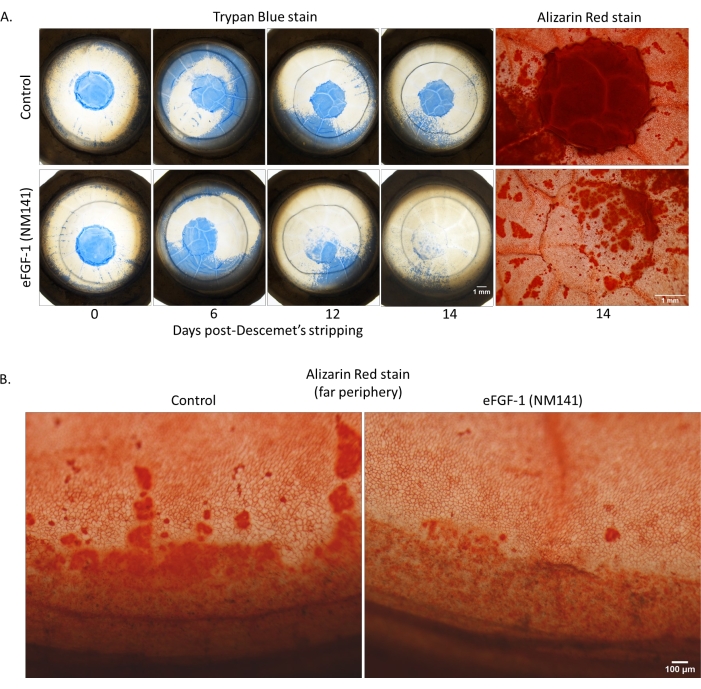
Figure 4: Vital dye staining of dystrophic corneas with peripheral staining. (A) Trypan staining of the intact Descemet's membrane can be seen advancing toward the center, then clearing over time in both control and treated corneas. (B) Alizarin patterns around the limbus represent a typical observation of peripheral damage and disorganized re-establishment of the endothelium seen in many donor corneas-in this case dystrophic. Please click here to view a larger version of this figure.
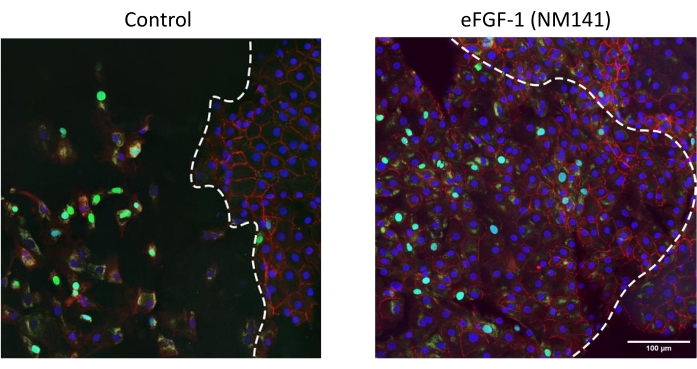
Figure 5: Stimulation of migration and proliferation of CECs by eFGF1 (NM141) in stripped dystrophic corneas. Confocal micrographs of Descemet's stripped corneas stained for ZO-1 (red), EdU (green), and Hoechst 33342 (blue). The dotted line represents the lesion edge with the stripped area on the left side of the image and the intact Descemet's membrane on the right. Please click here to view a larger version of this figure.
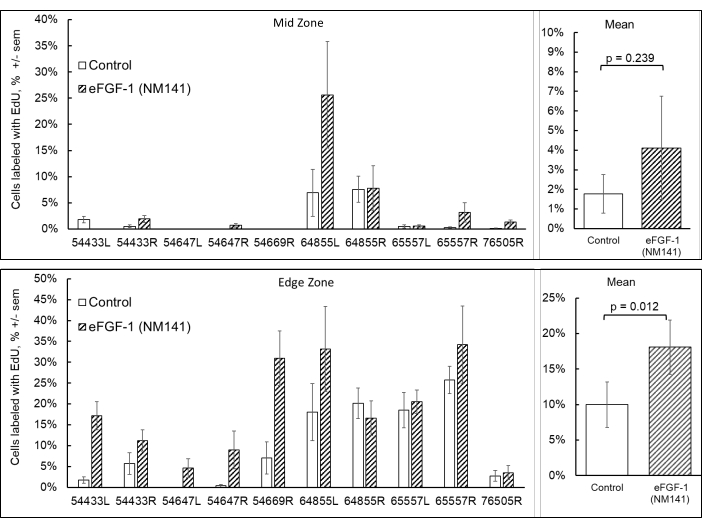
Figure 6: Quantitation of proliferating cells from normal corneas quartered using a scalpel and incubated in media containing EdU with or without eFGF-1 (NM141) for 48 h. Quarters were imaged in triplicate at the undisturbed mid zone and along the cut edge, and counts averaged to determine the percentage of cells incorporating EdU in the mid and edge zones. Figure modified from Journal of Ocular Pharmacology and Therapeutics, used with permission16. Please click here to view a larger version of this figure.
Supplemental Figure 1: Normal human corneas show significantly greater healing from DSO when cultured with eFGF1 (NM141). ImageJ was used to measure the stained areas and determine % healing. Please click here to download this File.
Discussion
Many ophthalmologists have concerns about recommending DSO to their patients for two main reasons: 1) the lengthy healing process, and 2) lack of data (DSO is a new concept in the field of ophthalmic surgery). The research we have presented would be of great utility for easing both of these concerns. Based on data from this study and others, the FDA has approved a Phase 2 clinical trial where eFGF1 (NM141) will be administered at varying dosing schedules to patients undergoing DSO17.
The method described above was modeled after a study performed by Soh et al., where corneal endothelial healing was evaluated with and without the ROCK inhibitor Y-27632 in both scratched and peeled wounds18. While Y-27632 accelerated endothelial regeneration with Descemet's membrane still intact, no substantial healing was found in stripped wounds even with treatment. Using a similar stripping technique followed by treatment with or without eFGF1 (NM141), the observations we found were not consistent with those of Soh and colleagues. The absence of Trypan Blue staining in many treated corneas at Day 14, and the presence of ZO-1 positive tight junctions within the reformed endothelial layer argues that an intact barrier, part of the CEC's natural function, was restored in both normal and dystrophic corneas. Though not quantified in this study, the presence of EdU-positive cells in and around the stripped area also suggests proliferation as a healing mechanism, one which we have previously established can be stimulated by eFGF1 (NM141) in injured corneas16. Statistical analysis showed treatment with eFGF1 (NM141) resulted in significantly greater healing from DSO, on average over twice as high as control corneas at the 14 day timepoint. Though healing rates vary moderately between individuals-a typical characteristic of donor corneas-the results' replicability over large sample sizes also evidences a highly measurable method. To our knowledge, there are no other examples of an ex vivo Descemet's stripping model in the literature.
Key components of the protocol itself that would serve valuable to other researchers studying DSO are the use of Trypan Blue to detect bare stroma, and the image processing technique used to measure the stained area. Trypan Blue is commonly used in ophthalmic surgery, particularly when working with Descemet's membrane to detect non-viable cells and assist in visibility of the tissue. The staining timepoints included in this protocol allowed for effective repeat staining without over-exposing corneas to Trypan Blue, as it has shown to be toxic to CECs at high concentrations19. The reduction of stained area over 14 days in all corneas, confirmed by Alizarin Red and immunohistochemistry to be the result of migrated CECs, demonstrates a simple and reproducible method to measure healing. Using ImageJ's color threshold menu, multiple analysts collected data with standard deviations consistently below 1% (data not shown). Though alternative programs may perform similarly, ImageJ is an open-source software capable of producing accurate area measurements to track healing.
There is, however, one aspect of the stripping protocol that we found to be both necessary for wound creation, yet obstructive to the overall healing process. Using a sharp, 30 G needle to score Descemet's membrane along the mark left by the biopsy punch enables the creation of a smooth, circular wound which is noted by clinicians to support faster healing10. At the same time, this step is damaging to the cornea, as it can create tears in the stromal fibers causing stromal cell death, impeding migration of endothelial cells across the wound edge, and inducing the formation of nodules that result in more persistent post-operative edema20. Clinicians performing DSO typically use a reverse Sinskey hook to initiate the wound, but without any intraocular pressure keeping the cornea taut, this tool is less effective in the ex vivo model. An alternative tool capable of tearing Descemet's membrane without damaging the underlying stroma would improve upon the protocol, for example the irrigation and aspiration handpiece recommended by Macsai and Shiloach10. Further experimentation will be needed to determine whether this technique is compatible with the ex vivo model.
A challenge which appears inherent to the ex vivo model is the frequent occurrence of CEC death in the area peripheral to the wound, particularly in dystrophic corneas. This occasionally obscured quantitation of the wound area, since the accuracy of the color thresholding tool becomes more limited as the stained area extends beyond the center of the cornea where its curvature results in uneven light distribution. However, these variable measurements primarily occurred at earlier timepoints before peripheral staining gradually receded as damaged CECs cleared and neighboring cells stretched, migrated, or proliferated to replace them. By the final 14 day timepoint, the stained area had localized back to the center of the cornea, and all images were measurable. A similar observation was made with comparable frequency by Soh et al., where five of 14 normal corneas presented what they termed 'Premature Culture Failure' (PCF) early in the culture period18. While the damage reversed itself over time in our corneas and yet was permanent in their case, this may be attributed to the fact that their method required wounding a larger area of the cornea. The observation of more prevalent peripheral Trypan staining in dystrophic corneas may indicate that dystrophic corneas are more susceptible to endothelial cell death than healthy corneas. While the exact cause of this cell death has yet to be elucidated, we believe it is unlikely that this issue will be relevant to human corneas in vivo. Damage to the peripheral endothelium has not been reported in any clinical case studies of DSO to our knowledge, suggesting that this phenomenon is unique to donor corneas cultured ex vivo6,8,10,11,21. Aside from two instances where only control corneas stained, all observations of peripheral staining were paired, so it is unlikely that the cause was exposure to eFGF1 (NM141). However, it is possible that in those instances treatment may have provided a protective effect against the damage that otherwise would have induced peripheral staining in both corneas. Further investigation on this hypothesis is needed.
Another limitation to this method is sourcing donor corneas representative of the FECD phenotype for which DSO is intended. Donor corneas of any kind are scarce, hence the need for a surgery that avoids the use of donor tissue. For our purposes, the only corneas available are those rejected from transplant for various reasons. Eye banks further classify these corneas as normal or dystrophic based on criteria including presence of guttae, low or un-measurable ECD, and irregular CEC morphology. Confirming a dystrophic diagnosis prior to accepting tissue from an eye bank is also nearly impossible, since most donors' medical history does not include past ocular history and the only information provided is the ECD value, the technician's notes, and in some cases a representative specular image. The dystrophic corneas obtained for this study did not show confluent central guttae upon confocal microscopy performed after the culture period was complete, suggesting that they may represent "early" stages of FECD. We do not expect this to have a significant impact on the implications of the study, as the purpose of DSO is to remove confluent areas of guttae, allowing healthy peripheral cells to migrate inward.
This method provides a highly applicable and reproducible technique to evaluate agents that might impact CEC proliferation and migration. The model has several features that make it more physiologically relevant than in vitro models that involve cultured CECs, even when seeded onto human corneal tissue graft22,23,24. First, the CECs to be stimulated are in a monolayer exactly as they exist in the patient's eye, and are migrating across the corneal stroma as they would after clinical DSO. The stroma in question is from the same patient as the CECs, thus controlling for potential FECD-related stromal differences. There is no need to explant, dissociate, and expand cultures of CECs with the associated challenges and potential for Endothelial to Mesenchymal Transition (EnMT) during the culturing process. The protocol described is itself very similar to the clinical DSO procedure. While it bypasses the culture and expansion steps, this method does have the limitation that the duration of study is restricted by corneal swelling, as the epithelial layer is not maintained. This inhibits us from investigating the morphology of CECs that have migrated to cover the stripped area, leaving it unclear whether they will eventually rearrange into a hexagonal array in this model. Garcin et al. have developed one potential solution with their active storage machine (ASM), a device shown to keep corneas in culture for up to 3 months with significantly less edema than corneas maintained in traditional organ culture25. Such a device may be helpful in replicating and expanding upon this work.
This model has potential utility in testing other wound healing therapies (e.g., ROCK inhibitors), evaluating modifications to surgical technique and comparing healing across different donor populations or stages of disease. We hope this research, in conjunction with clinical trial data as it comes out, encourages clinicians to consider DSO as a valuable treatment option for their eligible FECD patients.
Disclosures
DDE, SP, GD, and JW are employees of and hold equity in Trefoil Therapeutics, Inc. DDE is inventor on patents covering eFGF1 (NM141).
Acknowledgements
Funding for this work was supported by Trefoil Therapeutics and NIH NCATS TRND CRADA #2016-04. The authors would like to thank Tony Wong for histopathology advice and services, the Nikon Imaging Center at UC San Diego for use of their confocal microscope, and Drs. Natalie Afshari and Marian Macsai for their advice on surgical technique. In addition, the authors extend their gratitude to the donors of eyes and the eye banks for providing corneas.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2µm sterile 1000 mL filter units | VWR | 10040-440 | |
| 0.2µm sterile 250 mL filter units | VWR | 10040-464 | |
| 0.2µm sterile 500 mL filter units | VWR | 10040-436 | |
| 10mL syringe Luer-Lok Tip | Becton Dickinson | 302995 | |
| 12 well tissue culture treated plate | Corning | 3513 | |
| 15 mL conical Tubes | VWR | 89039-668 | |
| 16% paraformaldehyde (PFA) | Electron Microscopy Science | 15710 | |
| 2mL aspirating pipette | VWR | 414004-265 | |
| 310 direct heat CO2 incubator | Forma Scientific | 13-998-082 | Set to 37°C, 6% CO2 |
| 50 mL conical tubes | VWR | 89039-660 | |
| 5-Ethynyl-2'-deoxyuridine (EdU) | Thermo Scientific | C10337 | |
| 5mL, 10mL, 25mL and 50mL serological pipettes | VWR | 89130-896, -898, -900, -902 | |
| 6 well tissue culture treated plate | Corning | 3516 | |
| 70% ethanol | BDH | BDH1164-4LP | |
| Alexa Fluor 488 azide | Thermo Scientific | A10266 | |
| Alizarin Red S | Sigma | A5533-25G | |
| Analytical balance | Sartorious | R200D | |
| Antibiotic & Antimycotic 100x (anti-anti) | Thermo Scientific | 15240-062 | |
| Anti-magnetic stainless steel forceps | Excelta | 7-SA | |
| Bottle top dispenser | Ward's Science | 470134-946 | |
| Bovine serum albumin (BSA) | Fisher Scientific | BP9700-100 | |
| Calcium chloride (CaCl) | Amresco | 1B1110-500G | |
| Chex-all II sterilzation pouches | Propperman | 24008 | |
| Cirpofloxacin hydrochloride | Alfa Aesar | J61970 | |
| Copper (II) sulfate pentahydrate (CuSO4) | Sigma | 469130-50g | |
| Dissecting microscope | Nikon | SMZ1270 | |
| Dry vacuum pump | Welch | 2019B-01 | |
| Fetal bovine serum (FBS) | Thermo Scientific | A31606-01 | |
| Frosted micro slides | VWR | 48311-703 | |
| Galaxy miniStar microcentrifuge | VWR | C1413, VWR | |
| Goat anti-mouse IgG (H+L) secondary antibody, Alexa Fluor Plus 555 | Thermo Scientific | A32727 | |
| Goat serum | Sigma | G9023 | |
| Haemo-Sol detergent | Haemo-Sol International LLC | 026-050 | |
| Hoechst 33342, trihydrochloride, trihydrate | Thermo Scientific | H3570 | |
| Hot plate/stirrer | Corning | PC-320 | |
| Human corneas | Lions Eye Institute for Transplant and Research, Advancing Sight Network, Eversight Eye Bank, Lions Vision Gift, and Georgia Eye Bank | NA | |
| Hydrochloric acid (HCl) | BDH | BDH7204 | |
| ImageJ | National Institute of Health | Version 1.52a | |
| Infinity 3s microscopy camera | Lumenera | 1URCAP2 | |
| Infinity analyze software | Lumenera | Version 6.5.5 | |
| Insulin transferrin selenium (ITS) | Corning | 41400-045 | |
| Iris scissors, 11 cm | World Precision Instruments | 501264-G | |
| L- Ascorbic acid | Sigma | A4544-25G | |
| Manual single channel pipet | Rainin | 17014-392, -391, -382 | |
| Needle PrecisionGlide 30G | Becton Dickinson | 305106 | |
| N-Met141 TTHX1114 | Biopharmaceutical Development Program | NA | |
| Opti-Mem I + GlutaMAX-1 (Opti-MEM) | Thermo Scientific | 51985-034 | |
| Orion Star A211 pH meter | Thermo Scientific | STARA211 | |
| Petri dishes | VWR | 89107-632 | |
| Potassium chloride (KCl) | BDH | BDH9258-500G | |
| Potassium phosphate monobasic (KH2PO4) | VWR | 0781-500G | |
| Powerpette plus pipet controller | VWR | 75856-456 | |
| Precision water bath 188 | Precision Scientific Incorporated | WB05 | Set to 37°C |
| Purifier Class II model biosafety cabinet | Labconco | 36213043726 | |
| Safe-Lock tubes, 1.5 mL | Eppendorf | 22363212 | |
| Scalpel size 22 stainless steel | Sklar | 446479 | |
| Sodium chloride (NaCl) | VWR | 2041-2.5K | |
| Sodium hosphate dibasic (Na2HPO4) | VWR | 0404-1KG | |
| Standard shaker | VWR | 89032-092 | |
| Standard solid refrigerator | VWR | 10820-402 | Set to 4°C |
| Sterilmatic autoclave | Market Forge | STM-EL | |
| Syringe filters | VWR | 28145-477 | |
| Test tube rocker | Thermo Scientific | M48725Q | |
| Tru-Punch disposable biopsy punch, 4 mm | Sklar | 96-1146 | |
| Trypan Blue | Thermo Scientific | 15250-061 | |
| Tween-20 | Sigma | P7949-100mL | |
| Vibrance antifade mounting medium with DAPI | Vector Laboratories Inc. | H-1800 | |
| VistaVision cover glasses, no. 1 | VWR | 16004-098 | |
| Vortex Genie 2 | Fisher Scientific | G-560 | |
| ZO-1 monoclonal antibody (ZO1-1A12) | Thermo Scientific | 33-9100 |
References
- Eghrari, A. O., Gottsch, J. D. Fuchs' corneal dystrophy. Expert Review of Ophthalmology. 5 (2), 147-159 (2010).
- Ku, B., et al. Endothelial cell loss in penetrating keratoplasty, endothelial keratoplasty, and deep anterior lamellar keratoplasty. Taiwan Journal of Ophthalmology. 7 (4), 199 (2017).
- Gain, P., et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmology. 134 (2), 167-173 (2016).
- Braunstein, R. E., Airiani, S., Chang, M. A., Odrich, M. G. Corneal edema resolution after "descemetorhexis". Journal of Cataract and Refractive Surgery. 29 (7), 1436-1439 (2003).
- Pan, J. C., Eong, K. G. A. Spontaneous resolution of corneal oedema after inadvertent 'descemetorhexis' during cataract surgery. Clinical and Experimental Ophthalmology. 34 (9), 896-897 (2006).
- Shah, R. D., et al. Spontaneous corneal clearing after Descemet's stripping without endothelial replacement. Ophthalmology. 119 (2), 256-260 (2012).
- Ziaei, M., Barsam, A., Mearza, A. Spontaneous corneal clearance despite graft removal in Descemet stripping endothelial keratoplasty in Fuchs endothelial dystrophy. Cornea. 32 (7), 164-166 (2013).
- Huang, M. J., Kane, S., Dhaliwal, D. K. Descemetorhexis without endothelial keratoplasty versus DMEK for treatment of fuchs endothelial corneal dystrophy. Cornea. 37 (12), 1497 (2018).
- Davies, E., Jurkunas, U., Pineda, R. Pilot study of corneal clearance with the use of a rho-kinase inhibitor after descemetorhexis without endothelial keratoplasty for Fuchs endothelial corneal dystrophy. Cornea. 40 (7), 899-902 (2021).
- Macsai, M. S., Shiloach, M. Use of topical rho kinase inhibitors in the treatment of Fuchs dystrophy after Descemet stripping only. Cornea. 38 (5), 529-534 (2019).
- Moloney, G., et al. Descemetorhexis without grafting for Fuchs endothelial dystrophy-supplementation with topical ripasudil. Cornea. 36 (6), 642-648 (2017).
- Thalmann-Goetsch, A., Engelmann, K., Bednarz, J. Comparative study on the effects of different growth factors on migration of bovine corneal endothelial cells during wound healing. Acta Ophthalmologica Scandinavica. 75 (5), 490-495 (1997).
- Landshman, N., et al. Regeneration of cat corneal endothelium induced in vivo by fibroblast growth factor. Experimental Eye Research. 45 (6), 805-811 (1987).
- Lee, J., Blaber, M. Increased functional half-life of fibroblast growth factor-1 by recovering a vestigial disulfide bond. Journal of Proteins & Proteomics. 1, 37-42 (2010).
- Xia, X., et al. Engineering a cysteine-free form of human fibroblast growth factor-1 for "second generation" therapeutic application. Journal of Pharmaceutical Sciences. 105 (4), 1444-1453 (2016).
- Eveleth, D., Pizzuto, S., Weant, J., Jenkins-Eveleth, J., Bradshaw, R. Proliferation of human corneal endothelia in organ culture stimulated by wounding and the engineered human fibroblast growth factor 1 derivative TTHX1114. Journal of Ocular Pharmacology and Therapeutics: the Official Journal of the. 36 (9), 686-696 (2020).
- TTHX1114(NM141) in Combination With DWEK/DSO. ClinicalTrials.gov Available from: https://clinicaltrials.gov/ct2/show/NCT04676737?term=TTHX1114&draw=2&rank=2 (2020)
- Soh, Y. Q., et al. Predicative factors for corneal endothelial cell migration). Investigative Ophthalmology & Visual Science. 57 (2), 338-348 (2016).
- van Dooren, B. T. H., Beekhuis, W. H., Pels, E. Biocompatibility of trypan blue with human corneal cells. Archives of Ophthalmology. 122, (2004).
- Davies, E., Jurkunas, U., Pineda, R. Predictive Factors for Corneal Clearance After Descemetorhexis Without Endothelial Keratoplasty. Cornea. 37 (2), 736-742 (2018).
- Borkar, D. S., Veldman, P., Colby, K. A. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea. 35 (10), 1267-1273 (2016).
- Amano, S., Mimura, T., Yamagami, S., Osakabe, Y., Miyata, K. Properties of corneas reconstructed with cultured human corneal endothelial cells and human corneal stroma. Japanese Journal of Ophthalmology. 49 (6), 448-452 (2005).
- Rolev, K., OʼDonovan, D. G., Coussons, P., King, L., Rajan, M. S. Feasibility study of human corneal endothelial cell transplantation using an in vitro human corneal model. Cornea. 37 (6), 778-784 (2018).
- Spinozzi, D., et al. In vitro evaluation and transplantation of human corneal endothelial cells cultured on biocompatible carriers. Cell Transplantation. 29, 1-11 (2020).
- Garcin, T., et al. Three-month storage of human corneas in an active storage machine. Transplantation. 104 (6), 1159-1165 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved