Method Article
Robotic Cochlear Implantation for Direct Cochlear Access
In This Article
Summary
Robotic cochlear implantation is a procedure for minimally invasive inner ear access. Compared to conventional surgery, robotic cochlear implantation involves additional steps that need to be carried out in the operating room. In this article we give a description of the procedure and highlight the important aspects of robotic cochlear implantation.
Abstract
Robot-assisted systems offer great potential for gentler and more precise cochlear implantation. In this article, we provide a comprehensive overview of the clinical workflow for robotic cochlear implantation using a robotic system specifically developed for a minimally invasive, direct cochlear access. The clinical workflow involves experts from various disciplines and requires training to ensure a smooth and safe procedure. The protocol briefly summarizes the history of robotic cochlear implantation. The clinical sequence is explained in detail, beginning with the assessment of patient eligibility and covering surgical preparation, preoperative planning with the special planning software, drilling of the middle ear access, intraoperative imaging to confirm the trajectory, milling of the inner ear access, insertion of the electrode array, and implant management. The steps that require special attention are discussed. As an example, the postoperative outcome of robotic cochlear implantation in a patient with advanced otosclerosis is presented. Finally, the procedure is discussed in the context of the authors' experience.
Introduction
A cochlear implant (CI) is the standard treatment for severe to profound sensorineural hearing loss1. The surgical procedure for cochlear implantation aims to atraumatically insert the cochlear implant electrode array into the cochlea. For the implantation, surgeons must provide access from the surface of the temporal bone to the cochlea. In conventional procedures, this access is created by removing portions of the mastoid bone through a mastoidectomy and posterior tympanotomy2.
Robot-assisted cochlear implantation aims to perform a minimally invasive access through a small tunnel to the inner ear for electrode array insertion. To date, several systems for robot-assisted cochlear implantation are in development or already available on the market. One such system provides robotic-controlled drilling of the mastoid and electrode insertion and has been recently evaluated in patients3. Another device is a patient-specific guiding system for tunnel drilling and electrode insertion4. Two systems that do not provide the inner ear access tunnel but rather the alignment and motorized insertion of electrode arrays have recently received medical device approval in Europe and the United States5,6. The first clinical implementation of a minimally invasive tunnel procedure using a stereotactic guidance frame was performed by Labadie et al.7. The first robotic system and planning software applied in clinical cases was developed through collaboration between the ARTORG Center for Biomedical Engineering at the University of Bern and the Department of Otolaryngology at Bern University Hospital in Switzerland8,9,10,11. The planning software and system were later commercialized by a spin-off company.
Here, the authors present the protocol involved in performing robotic cochlear implantation with a dedicated robotic cochlear implantation system. The aspects of selecting suitable patients, preoperative planning of the access tunnel, and the complete surgical procedure are covered and discussed. The aim of this article is to present an overview of the procedure and share the authors' experience with the system.
Protocol
This study was performed in compliance with institutional guidelines and was approved by the local institutional review board (ID 2020-02561). The patient gave written informed consent for further use of the images and videos. The video shows the processes involved in performing robotic cochlear implantation with the planning software and the robotic system (please refer to the Table of Materials for further details) according to the procedure described by the manufacturer.
1. Patient candidacy screening
NOTE: Use existing preoperative computed tomography images for this step. Currently, robotic cochlear implantation with the system used in this protocol is only available from a single manufacturer (see Table of Materials) for implant systems. Please refer to the instructions for use manual of the planning software for specific details regarding button clicks, software commands, and user inputs.
- Use the planning software to load preoperative computed tomography images and to generate the surfaces of the temporal bone, the external auditory canal, the ossicles, the facial nerve, the chorda tympani, and the cochlea.
- Use the planning software to plan a virtual trajectory through the facial recess.
- Confirm a safe distance between the drilling trajectory and the surrounding anatomical structures. To ensure a safe drilling trajectory, the distance of the trajectory to the facial nerve has to be at least 0.4 mm, and the distance to the chorda tympani has to be at least 0.3 mm. Only patients with safe drilling distances are eligible for robotic cochlear implantation.
- Use the planning software to select a suitable electrode array size. In cases with residual hearing, consider including the preoperative audiogram for CI electrode array selection.
2. Fiducial screw insertion
- Prepare the patient on the OR table and administer general anesthesia as per the conventional cochlear implantation procedure.
- Mark the retroauricular incision for the cochlear implant using a surgical marker. Perform the incision, lift the musculocutaneous flap, and use the curette to expose the mastoid cortical bone.
- Mark the position of the five fiducial screws. Position the first four registration screws retroauricularly at approximately 20-30 mm in a trapezoid pattern. Place the fifth screw, for patient marker attachment, approximately a thumb distance from the first four screws and as inferior as possible.
NOTE: Refer to the instructions for an illustration of the screw arrangement. - Use the pre-drill bit and drill handpiece to pre-drill the holes for the screws, with particular emphasis that the drill is held perpendicular to the bone surface.Insert the screws into the pre-drilled holes. Ensure that the screws are firmly fixed in the bone.
CAUTION: Always check the stability of the screws. In case of a loose screw, repeat step 2.3. and step 2.4. to reposition the screw.
3. Preoperative imaging
- Perform computed-tomography (CT) imaging or cone beam CT imaging with a minimum resolution of 0.2 mm x 0.2 mm x 0.2 mm as per the manufacturer's instructions. Perform imaging under apnea to reduce motion artifacts.
- Export the images via a USB stick and import them into the planning software. Verify the image data quality and visibility of all the screws in the CT data.
4. Preoperative planning
NOTE: Perform preoperative planning in parallel with patient preparation (step 5.) to save time. Please refer to the instructions for use manual of the planning software for mesh generation and for specific details regarding button clicks, software commands, and user inputs.
- Run the automatic fiducial screw detection in the planning software. Generate the temporal bone surface mesh.
- Generate the external auditory canal surface mesh. Generate the malleus and incus surface meshes.
- Generate the stapes surface mesh.
- Generate the facial nerve surface mesh.
CAUTION: Make sure that you segment the facial nerve with a safety margin (e.g., 3 voxels). If desired, let a neuroradiologist verify the labels. - Generate the chorda tympani surface mesh. Generate the cochlea surface mesh and specify the target position on the cochlea (usually the center of the round window).
- Plan the drill trajectory and approve the plan with the neuroradiologist. Export the plan to a data stick for transfer to the robotic system.
5. Patient preparation
- Align the patient's head within the headrest in a way that the neck is supported by the bottom cushion and the patient's nose is aligned with the center of the head rest's top frame. Ensure that the patient's head is sufficiently fixed in the headrest.
CAUTION: The correct alignment of the head is essential for the accessibility of the robotic system to the surgical site. - Place the facial nerve monitoring electrodes. Place the bipolar needle electrodes in the orbicularis oculi and the orbicularis oris for monitoring. Place the self-adhesive pad electrodes on the superficial facial nerve branch for stimulation. Place the monopolar needle electrodes on the chest for stimulation and monitoring.
- Test the correct placement of the electrodes by control stimulation performed according to the instructions for use from the manufacturer of the robotic system.
- Cover the robotic system and navigation platform with a sterile drape.
- Place, align, and fix the patient marker on the fifth screw such that it is visible to the robotic system's tracking camera. Ensure that the patient marker is attached rigidly and that all the joints are firmly tightened. It is critical to avoid any movement of the patient marker after the registration process. In the case of movement, repeat the registration.
- Perform patient-to-plan registration, which is the process to relate the virtual plan to the actual patient. Use the handpiece with the registration tool and place it onto each fiducial screw (four times). Perform the registration procedure as per the instructions for use from the manufacturer. The screen on the navigation platform indicates which screw to position the tool on.
- After all screw positions are digitized, the registration accuracy is computed. Confirm that the registration accuracy is sufficient to continue by checking the fiducial registration error (FRE). The robotic system does not allow the continuation of the procedure if the fiducial registration error (FRE) is higher than 0.050 mm.
6. Middle ear access - Phase 1
- Insert the drill bit into the handpiece and attach the irrigation nozzle. Move the robotic arm into the surgical field. The handpiece with the drill slowly approaches the surgical site. Confirm the alignment of the drill bit with the virtual trajectory planned in the planning software.
- Start drilling with the robotic system. The system will drill with a pecking motion until the first safety checkpoint (above the facial recess) is reached. After the first safety checkpoint is reached, move the robotic arm out of the surgical field.
7. Intraoperative imaging safety check
- Remove the patient marker from the patient. Insert and push the trajectory reference rod inside the drilled tunnel. Drape the patient's head with sterile draping.
- Perform CT imaging or cone beam CT imaging with the help of the neuroradiology department team.
- Load the CT data into the planning software and confirm together with the neuroradiologist that the trajectory is safe. Refer to the instructions for use of the planning software for further details. Remove the draping and the trajectory reference rod.
8. Middle ear access - Phase 2
- Reattach the patient marker. Ensure that the patient marker is attached rigidly and that all the joints are firmly tightened.
- Repeat the registration by placing the handpiece with the registration tool onto each of the fiducial screws for position digitization. After all the screw positions are digitized, the registration accuracy is computed. Confirm that the registration accuracy is sufficient to continue.
- Insert the drill bit into the handpiece. Confirm the alignment of the drill bit with the drill tunnel and continue drilling until the first facial nerve stimulation point is reached.
- Insert the facial nerve probe to check the integrity of the facial nerve. Afterward, the robotic system will drill to the next facial nerve stimulation point. In total, five facial nerve stimulation points will be tested.
- Continue drilling until the tympanic cavity is reached.
9. Inner ear access
NOTE: Inner ear access is a semi-automatic procedure that can be stopped by the surgeon at any time for visual inspection.
- Remove the drill bit from the handpiece of the robotic system and insert the diamond drill bit for inner ear access. Use the trajectory pointer to verify the target upon milling.
- Start the robotic system to mill the bony overhang. The system will automatically stop after breakthrough to ensure that a sufficient aperture for the electrode array is achieved, while aiming to preserve the round window membrane.
- Confirm the inner ear access either via an endoscope or via a microscope through a tympanomeatal flap.
10. Implant management and electrode insertion
- Remove the patient marker and all five fiducial screws. If not already performed, make a tympanomeatal flap to visualize the cochlear promontory.
- Mark the implant body position using the surgical template and prepare the implant pocket.
- Mill out the lead canal for the excess electrode lead using an otologic drill. Clean the drilled tunnel with suction and irrigation.
- Manually open the round window membrane with a pick. Insert the insertion guide tube into the drilled tunnel. The tube will ensure that the electrode array is protected against blood and bone dust and is directed to the inner ear access.
- Fix the cochlear implant body in the pocket and manually insert the electrode array through the insertion guide tube.
- Mark the electrode lead to indicate full insertion through the insertion guide tube. Use the insertion guide tube as a reference, i.e., by placing it next to the electrode array such that the medial end of the guide tube is aligned with the intended insertion depth, e.g., the outermost contact. Then, mark the array at the lateral end of the guide tube.
- After the final insertion depth is achieved, remove the insertion guide tube. Fix the electrode array with fat and arrange the excess electrode lead as a loop in the mastoid cavity as in conventional cochlear implantation.
11. Implant telemetry and wound closure
- Perform impedance telemetry and record electrically evoked compound action potentials for nerve response monitoring12.
- Close the wound using disposable stitches.
Results
Robotic cochlear implantation is particularly suitable for cases with difficult anatomical conditions. Here, the postoperative results in a patient with far advanced otosclerosis are presented. Figure 1 shows a preoperative CT image. The advanced state of otosclerosis has disintegrated the petrous bone, making the cochlea hardly discernible.
The postoperative outcome is illustrated in Figure 2. The small tunnel access can be seen. In this case, surgical planning was used to preoperatively identify an optimal insertion access to the inner ear. The successful insertion of the electrode array can be seen, with an angular insertion depth of about 270°.

Figure 1: Robotic cochlear implantation in a patient with far-advanced otosclerosis. Axial computed tomography slice of the left temporal bone shows the barely discernible cochlea (red ellipse). Please click here to view a larger version of this figure.
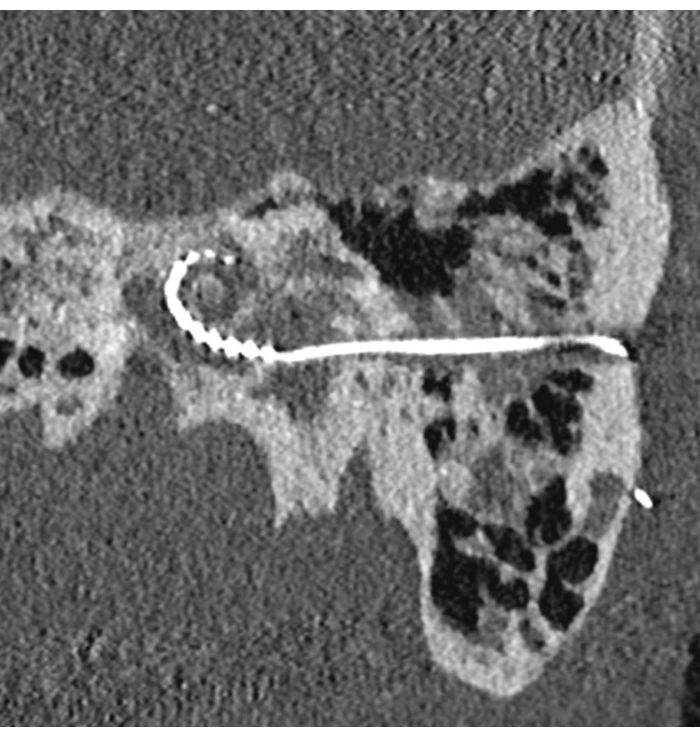
Figure 2: Robotic cochlear implantation in a patient with far-advanced otosclerosis. Postoperative image showing the drilled tunnel and the inserted electrode array. Please click here to view a larger version of this figure.
Discussion
Here, an overview of the steps involved in robotic cochlear implantation is presented. An important part is the selection of suitable candidates for the procedure. To ensure that the safety margins during surgery can be maintained, careful candidate screening needs to be performed to ensure eligibility for the procedure. The distance between the virtually planned trajectory and the facial nerve should be at least 0.4 mm. In addition, at least 0.3 mm distance to the chorda tympani needs to be available. To provide more flexibility in trajectory planning after preoperative imaging on the day of surgery, even larger limits can be considered for patient selection.
As the robotic system relies on the fiducial landmark screws to transfer the plan to the patient, they are of central importance for a safe procedure. The surgeon should carefully select the positions of the fiducial screws to ensure that enough space is available for trajectory drilling. A linear arrangement of three screws should be avoided. Also, it needs to be ensured that the screw for the patient marker is positioned such that the marker remains visible throughout the procedure. The instructions for use of the robotic system provide detailed guidelines for screw positioning. When placing the screws, it needs to be ensured that the holes are pre-drilled perpendicularly to the surface of the mastoid bone. Tight fixing of the screws ensures that no movement occurs during the procedure.
For preoperative imaging, patients should be scanned in apnea, as the breathing motion of the patient can cause motion artifacts that may not be immediately identifiable in the images but later on during the registration process can cause errors that impede commencing the procedure. It should be ensured that the person performing the preoperative planning has received extensive training to confidently identify and label the anatomical structures. In particular, the course of the facial nerve, the chorda tympani, and the selection of the target at the cochlea (usually the center of the round window membrane) need to be trained. For facial nerve generation, additional safety through over-segmentation of the nerve should be considered. In case no imaging modality is available directly in the operating room or no mobile imaging system can be transported into the operating room, the patient needs to be transferred to the neuroradiological department for imaging. The additional patient transfer time needs to be considered. Preoperative planning can be performed in parallel with patient transfer and preparation to save time.
The team should extensively train head positioning in the headrest to ensure that the patient marker and screws are visible to the system at later stages. Wrong head positing can result in invisibility of the markers or infeasible kinematics of the robotic arm. At all stages during robotic cochlear implantation, it needs to be ensured that all the screws are tightly fixed, the patient marker is rigidly attached, and the handpiece of the robot is fixed.
For intraoperative imaging using mobile imaging devices (e.g., mobile cone beam CT), sufficient clearance of the patient's head and the headrest with the sterile draping needs to be ensured. Motion artifacts caused by the scanner touching the sterile drape could worsen the image quality of the intraoperative image and impede decision-making on the safety of the drilled trajectory required for commencing of the drilling.
In an optimal case, the round window membrane is preserved after robotic inner ear access, sealing the inner ear from bone dust and blood that might be introduced by the consecutive steps involved in implant management. As the fiducial screws and patient reference marker are required for inner ear access, it is not recommended to prepare the implant bed before inner ear access to ensure sufficient space for screw placement. In case the round window membrane is not intact after inner ear access, the round window could be temporarily covered as a protective measure until the electrode array insertion is performed.
After access to the inner ear is established, the surgeon may use different techniques to visualize the access. Microscopic inspection through a tympanomeatal flap or direct endoscopic inspection are possible. However, for the later electrode array insertion, we recommend performing a tympanomeatal flap to provide direct access to the electrode array, if required13. The electrode array lead can be marked before insertion to indicate full insertions at the surface of the mastoid bone. We also recommend using the insertion guide tube during insertion to avoid contact with blood and bone dust and to constrain the electrode array to the insertion trajectory14.
The presented procedure applies task-autonomous robotics in the field of otological microsurgery. Potential advantages of the procedure include reproducible, minimally invasive access to the cochlea and, ultimately, targeted, and accurate insertion of electrodes, which could expand the pool of CI patients in the future. The current limitations of the system are the associated additional costs for material and trained staff, the longer surgical duration, and the still manually performed electrode insertion. Currently, robotic cochlear implantation requires more time (about 4 h) than conventional cochlear implantation (about 1.5 h). Therefore, the condition of the patient should be also considered for eligibility.
Disclosures
The authors declare that they have no conflicts of interest. This study was funded by the Department of Otorhinolaryngology, Head and Neck Surgery, at the Inselspital Bern.
Acknowledgements
The authors thank Gianni Pauciello, Department of Otorhinolaryngology, Head and Neck Surgery, Inselspital, Bern University Hospital, for video production and photography. We also thank Dr. Stefan Henle and the team of the Department of Anesthesiology and Pain Medicine, Inselspital, Bern University Hospital and the team at the Department of Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, Bern, Switzerland.
Materials
| Name | Company | Catalog Number | Comments |
| Cochlear implant | MED-EL, Austria | CE-labelled | |
| HEARO Consumable Set | CAScination, Switzerland | REF 50176 | CE-labelled |
| HEARO Instrument Set | CAScination, Switzerland | REF 30123 | CE-labelled |
| HEARO System Components | CAScination, Switzerland | CE-labelled | |
| Mobile cone beam CT scanner | XORAN Xcat | if not availalbe, imaging needs to be performed in the neuroradiological department | |
| OTOPLAN | CAScination, Switzerland | REF 20125 | CE-labelled |
| Planning laptop | Any computer with enough performance is suitable, software OTOPLAN installed | ||
| USB Stick | A surgical plan that was created with OTOPLAN is transferred to the HEARO system via a USB flash drive. |
References
- Wimmer, W., Weder, S., Caversaccio, M., Kompis, M. Speech intelligibility in noise with a pinna effect imitating cochlear implant processor. Otology & Neurotology. 37 (1), 19-23 (2016).
- Lenarz, T. Cochlear implant - State of the art. GMS Current Topics in Otorhinolaryngology -Head and Neck Surgery. 16, (2018).
- Klopp-Dutote, N., Lefranc, M., Strunski, V., Page, C. Minimally invasive fully ROBOT-assisted cochlear implantation in humans: Preliminary results in five consecutive patients. Clinical Otolaryngology. 46 (6), 1326-1330 (2021).
- Kluge, M., Rau, T., Lexow, J., Lenarz, T., Majdani, O. Untersuchung der Genauigkeit des RoboJig für die minimal-invasive Cochlea-Implantat-Chirurgie. Laryngo-Rhino-Otologie. 97, 10602 (2018).
- Barriat, S., Peigneux, N., Duran, U., Camby, S., Lefebvre, P. P. The use of a robot to insert an electrode array of cochlear implants in the cochlea: A feasibility study and preliminary results. Audiology and Neurotology. 26 (5), 361-367 (2021).
- . Clinical Trials.gov Available from: https://clinicaltrials.gov/ct2/show/NCT04577118 (2022)
- Labadie, R. F., et al. Minimally invasive image-guided cochlear implantation surgery: First report of clinical implementation. The Laryngoscope. 124 (8), 1915-1922 (2014).
- Caversaccio, M., et al. Robotic middle ear access for cochlear implantation: First in man. PLOS One. 14 (8), 0220543 (2019).
- Weber, S., et al. Instrument flight to the inner ear. Science Robotics. 2 (4), 4916 (2017).
- Bell, B., et al. In vitro accuracy evaluation of image-guided robot system for direct cochlear access. Otology & Neurotology. 34 (7), 1284-1290 (2013).
- Caversaccio, M., et al. Robotic cochlear implantation: Surgical procedure and first clinical experience. Acta Oto-Laryngologica. 137 (4), 447-454 (2017).
- Dillier, N., et al. Measurement of the electrically evoked compound action potential via a neural response telemetry system. The Annals of Otology, Rhinology, and Laryngology. 111, 407-414 (2002).
- Wimmer, W., et al. Cone beam and micro-computed tomography validation of manual array insertion for minimally invasive cochlear implantation. Audiology and Neuro-Otology. 19 (1), 22-30 (2014).
- Wimmer, W., et al. Electrode array insertion for minimally invasive robotic cochlear implantation with a guide tube. International Journal of Computer Assisted Radiology and Surgery. 11, 80-81 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved