A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Visualizing the Conformational Dynamics of Membrane Receptors Using Single-Molecule FRET
In This Article
Summary
This study presents a detailed procedure to perform single-molecule fluorescence resonance energy transfer (smFRET) experiments on G protein-coupled receptors (GPCRs) using site-specific labeling via unnatural amino acid (UAA) incorporation. The protocol provides a step-by-step guide for smFRET sample preparation, experiments, and data analysis.
Abstract
The ability of cells to respond to external signals is essential for cellular development, growth, and survival. To respond to a signal from the environment, a cell must be able to recognize and process it. This task mainly relies on the function of membrane receptors, whose role is to convert signals into the biochemical language of the cell. G protein-coupled receptors (GPCRs) constitute the largest family of membrane receptor proteins in humans. Among GPCRs, metabotropic glutamate receptors (mGluRs) are a unique subclass that function as obligate dimers and possess a large extracellular domain that contains the ligand-binding site. Recent advances in structural studies of mGluRs have improved the understanding of their activation process. However, the propagation of large-scale conformational changes through mGluRs during activation and modulation is poorly understood. Single-molecule fluorescence resonance energy transfer (smFRET) is a powerful technique to visualize and quantify the structural dynamics of biomolecules at the single-protein level. To visualize the dynamic process of mGluR2 activation, fluorescent conformational sensors based on unnatural amino acid (UAA) incorporation were developed that allowed site-specific protein labeling without perturbation of the native structure of receptors. The protocol described here explains how to perform these experiments, including the novel UAA labeling approach, sample preparation, and smFRET data acquisition and analysis. These strategies are generalizable and can be extended to investigate the conformational dynamics of a variety of membrane proteins.
Introduction
The transfer of information across the plasma membrane is heavily dependent on the function of membrane receptors1. Ligand binding to a receptor leads to a conformational change and receptor activation. This process is often allosteric in nature2. With over 800 members, G protein-coupled receptors (GPCRs) are the largest family of membrane receptors in humans3. Due to their role in nearly all cellular processes, GPCRs have become important targets for therapeutic development. In the canonical model of GPCR signaling, agonist activation results in conformational changes of the receptor that subsequently activate the heterotrimeric G protein complex via exchange of GDP for GTP at the nucleotide binding pocket of Gα. The activated Gα-GTP and Gβγ subunits then control the activity of downstream effector proteins and propagate the signaling cascade4,5. This signaling process essentially depends on the ability of ligands to change the three-dimensional shape of the receptor. A mechanistic understanding of how ligands achieve this is critical for developing new therapeutics and designing synthetic receptors and sensors.
Metabotropic glutamate receptors (mGluRs) are members of the class C GPCR family and are important for the slow neuromodulatory effects of glutamate and tuning neuronal excitability6,7. Among all GPCRs, class C GPCRs are structurally unique in that they function as obligate dimers. mGluRs contain three structural domains: the Venus flytrap (VFT) domain, cysteine-rich domain (CRD), and transmembrane domain (TMD)8. The conformational changes during the activation process are complex and involve local and global conformational coupling that propagate over a 12 nm distance, as well as dimer cooperativity. The intermediate conformations, temporal ordering of states, and rate of transition between states are unknown. By following the conformation of individual receptors in real time, it is possible to identify the transient intermediate states and the sequence of conformational changes during activation. This can be achieved by applying single-molecule fluorescence resonance energy transfer9,10 (smFRET), as was recently applied to visualize the propagation of conformational changes during the activation of mGluR211. A key step in FRET experiments is the generation of FRET sensors by site-specific insertion of the donor and acceptor fluorophores into the protein of interest. An unnatural amino acid (UAA) incorporation strategy was adopted12,13,14,15 to overcome the limitations of typical site-specific fluorescent labeling technologies that require the creation of cysteine-less mutants or the insertion of a large genetically encoded tag. This allowed the conformational rearrangement of the essential compact allosteric linker, which joined the ligand-binding and signaling domains of mGluR2, to be observed. In this protocol, a step-by-step guide to performing smFRET experiments on mGluR2 is presented, including the approach for site-specific labeling of mGluR2 with UAA to attach fluorophores using the copper-catalyzed azide cyclization reaction. Moreover, this protocol describes the methodology for the direct capture of membrane proteins and data analysis. The protocol outlined here is also applicable to studying the conformational dynamics of other membrane proteins.
Protocol
The overall workflow of the protocol is described in Figure 1.
1. Preparation of the sample chamber
- Slide and coverslip cleaning
NOTE: These steps aim to clean the surfaces of the slides as well as the coverslips and prepare them for aminosilanization. One critical requirement for conducting single-molecule fluorescence experiments on surface-tethered molecules is a passivated surface. The most reliable and reproducible passivation technique involves covalently attaching inert polymer chains to the glass surface as a dense layer. Polyethylene glycol (PEG) is the most efficient polymer used for surface passivation16. The details of the passivation procedure using PEG (PEGylation) are described below:- Mark holes to be drilled on the slides with a marker (~6 mm apart and away from the edge). Use a Dremel to drill small holes (1 mm diameter) on the glass slide. Immerse the slides in water during the drilling process.
- Wash the slides with acetone to remove residual ink from the marker.
- Remove from acetone and rinse the slides with water, then microwave them for 5 min in water at high power (700 W).
- Clean the slides with water before placing them in a glass staining jar to be sonicated. Place the coverslips in a different staining jar.
- Sonicate the slides and coverslips in acetone for 30 min in a bath sonicator at 23 °C.
- In the meantime, clean a glass flask for preparing the aminosilanization solution used during the next step. Fill the flask with 1 M KOH, sonicate the flask for 30 min, thoroughly rinse out the KOH with water, and then sonicate for an additional 30 min in methanol. Leave the flask in methanol until the time of the aminosilanization step.
- In the meantime, remove the aminosilane, mPEG, and biotin-PEG from the freezer (−20 °C) and allow them to come to room temperature (RT) in the dark.
- Dispose of acetone from the slide and coverslip jars in the appropriate chemical waste container, rinse thoroughly with water, and then sonicate the slides in 5 M KOH for 30 min.
- Rinse the slides and coverslips with water, and then sonicate in methanol for 2 min (repeat twice). Leave the jars filled with methanol until the aminosilanization step.
NOTE: In this study, deionized water is used unless otherwise mentioned. A bath sonicator working at 23 °C is used.
- Aminosilanization
NOTE: This step aims to covalently link aminosilane to the clean slide and coverslip surface. Functionalized mPEG and biotin-PEG will covalently bind to this surface in the next step.- Prepare an aminosilanization mixture in a clean flask (step 1.6). Prepare the solution by mixing methanol (150 mL), acetic acid (7.5 mL, use glass pipette), and aminosilane (2.5 mL).
- Mix the solution gently in the chemical fume hood, then pour it into the slide and coverslip jars. Use 50 mL for the coverslips and 100 mL for the slides. Ensure slides and coverslips are fully immersed in the solution.
- Incubate for 10 min, then sonicate the jars for 1 min (sonication removes the impurities from the surface), and incubate for another 10 min.
- Dispose of aminosilanization solution in the waste collection container. Add methanol to the jars, close the jars with lids, and shake gently by hand. Dispose of the methanol, and fill jars with water.
- Return the aminosilane bottle to the freezer (−20 °C).
- PEGylation
NOTE: These steps describe the PEGylation procedure.- During aminosilanization, prepare the pegylation buffer. Weigh 84 mg of sodium bicarbonate (NaHCO3) and add it to 10 mL of water (10 mM). In addition, weigh mPEG and biotin-PEG and set them aside. For six slides and coverslips, use 96 mg of mPEG and 1.2-2.4 mg of biotin-PEG.
NOTE: It is important not to add too much biotin-PEG since it can increase the number of background spots. Do not dissolve the PEG mixture until right before application to slides. - Rinse the slides with water, dry them with a gentle air blow, and then place them in the humidified assembly boxes.
NOTE: It is essential to use a clean airline. Avoid using compressed canned air, which can leave residues on the glass. - Add pegylation buffer to PEG powder mixture and pipette up and down gently multiple times to dissolve. Add 55 µL of pegylation buffer per slide (for six slides, add 330 µL of pegylation buffer).
- Centrifuge at 9,600 x g for 1 min at 23 °C to precipitate undissolved particles. Collect the supernatant to use in the next step.
NOTE: Biotin-PEG hydrolyzes quickly due to the presence of the NHS group. It is important to do the mixing and centrifugation steps quickly. - Apply 60 µL of PEGylation solution to each slide and then place the coverslip on top so that the PEGylation solution is sandwiched between the slide and the coverslip.
NOTE: Avoid introducing bubbles between the slide and coverslip, as this will reduce the passivation efficiency. Remove any bubbles by adjusting the coverslip and the slide with a pipet tip. - Place the slides in a drawer that is flat and dark. Slides can be stored overnight; however, incubation for 4-6 h results in optimum passivation.
NOTE: It is essential to remember which side is pegylated. - Mark the non-pegylated side before storage. After incubation, gently disassemble and rinse the slides and coverslips thoroughly with water
- Dry the slides and coverslips by blowing air. Keep the slides and coverslips in a sterile tube (50 mL), with the PEGylated surface facing away from each other. Store at −20 °C until the day of the experiment.
NOTE: It is best to use slides and coverslips within 4 weeks of preparation. The PEGylated surfaces should face away from each other. Storing the PEGylated slides and coverslips in vacuum-sealed bags can increase their shelf life.
- During aminosilanization, prepare the pegylation buffer. Weigh 84 mg of sodium bicarbonate (NaHCO3) and add it to 10 mL of water (10 mM). In addition, weigh mPEG and biotin-PEG and set them aside. For six slides and coverslips, use 96 mg of mPEG and 1.2-2.4 mg of biotin-PEG.
2. mGluR2 expression with incorporated unnatural amino acid, fluorescent labeling, and extraction
NOTE: This protocol outlines the preparation, reagents, and treatment of cells for expressing mGluR2 containing the UAA 4-azido-L-phenylalanine (AZP). The procedure is for HEK293T cells grown on 18 mm glass coverslips. The procedure can be scaled up as necessary.
- Seeding
NOTE: Maintain the HEK293T cells in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 unit·mL−1 penicillin-streptomycin, and 15 mM HEPES buffer (Supplementary File 1) (pH 7.4) at 37 °C under 5% CO2.- Passage the cells with 0.05% trypsin-EDTA. Seed HEK293T cells on poly-L/D-lysine (PLL/PDL) glass coverslips so that they reach ≥80% confluency during the time of transfection.
- Transfection
- Prepare a 40 mM stock solution of AZP in 0.1 M NaOH.
- Prepare AZP-supplemented media for growing cells and mGluR2 expression. Supplement standard media (+ FBS, pen/strep, 15 mM HEPES) using 40 mM AZP stock solution. Bring the final AZP concentration to 0.6 mM. Add 1 M HEPES solution (half the volume of 40 mM AZP stock solution added). For example, to prepare 10 mL of AZP supplemented media, combine 9.775 mL of standard media, 150 µL of 40 mM AZP stock solution, and 75 µL of 1 M HEPES solution.
- Filter (sterilize) the media using a syringe filter (0.2 µm, PES).
- Replace the standard media with media containing AZP-supplemented media prior to transfection.
NOTE: Be careful not to dry the cells during the replacement of the media. - Transfect the cells with transfection reagent (Table of Materials) following the manufacturer's manual. Transfect HEK293T cells on an 18 mm coverslip using a total of 2 µg of DNA (1000 ng of tRNA/synthetase + 1000 ng of amber codon-containing protein plasmid). Refer to Table 1 for the concentration and volume of components used.
- Change the media 24 h after transfection to fresh AZP-supplemented media and allow the cells to grow for an additional 24 h.
- Labeling with alkyne cyanine dyes
- 20 min before labeling, wash the coverslips with warm (37 °C) recording buffer (RB) (Supplementary File 1) twice, and move them to warm (37 °C) standard media with no AZP (+ FBS, Pen/Strep, 15 mM HEPES).
- Prepare the labeling solution containing Cy3-alkyne, Cy5-alkyne, BTTES, copper (II) sulfate (CuSO4), (+) sodium L-ascorbate, and aminoguanidine.
- Follow the order of making and adding solutions (Table 2):
- Prepare 50 mM BTTES.
- Prepare 100 mM aminoguanidine.
- Prepare 100 mM Na-ascorbate.
- Prepare 655.5 µL of RB.
- Add Cy3/Cy5 alkyne dye (10 mM in DMSO stock) to the RB.
NOTE: Add aminoguanidine to the RB. - Prepare 20 mM CuSO4.
- Mix CuSO4 and BTTES in a new tube (the solution will turn blue).
- Add the CuSO4 and BTTES mixture to RB (2.3.3.6.)
- Add the Na-Ascorbate.
- Follow the volume given in Table 2 below for an 18 mm coverslip.
- Mix the solution thoroughly and incubate on ice and in the dark for 10 min prior to labeling cells.
- Before adding the labeling solution to the coverslips, remove the media and wash them with RB. Add the labeling solution and incubate for 15 min at 37 °C in dark conditions.
- NOTE: To improve labeling, add glutamate (final concentration ~0.5 mM) after 10 min and incubate for an additional 5 min. Copper is very toxic to the cells, and the labeling reaction should not continue for more than 15 min in vivo. Prepare all the components fresh. Add Na-Ascorbate last. Keep the reaction at 4°C while preparing. However, upon the addition of labeling solution, the cells are to be stored at 37 °C inside the incubator.
- Harvesting the cells and extracting the proteins (cell lysis)
- Remove the labeling solution and wash the coverslip (18 mm) containing the mGluR2 transfected cells twice with the RB.
- Using a pipet, wash the cells off the coverslip and resuspend in RB (1 mL).
NOTE: Minimize the sample exposure to light as much as possible after this point. - Pellet the cells by spinning at 1,000 x g at 4 °C for 5 min and remove the supernatant. Resuspend the cell pellet in 80-130 µL of the lysis solution.
NOTE: The cell pellet should be visible by eye. The lysis volume depends on the amount of sample lost during the labeling and washing process. - Mix gently by pipetting to break up the pellet. Wrap in foil and place on the rocker at 4 °C for 0.5-1 h to lyse the cells.
- Pellet the insoluble fraction by centrifugation at 20,000 x g and 4 °C for 20 min. Transfer the supernatant to a fresh cold tube (the lysed protein that contains the fluorescently tagged protein of interest) and store it on ice for experiments.
3. Single-molecule flow chamber assembly and functionalization
- Remove the slide and the coverslip from the freezer and allow them to warm at RT in darkness (~30 min).
- Assemble the chamber using double-sided tape by sandwiching strips of double-sided tape between the slide and coverslip. Make sure the PEGylated surfaces form the interior of the flow chamber.
- Using a pipet tip, press the coverslip to ensure the tape is making complete contact with both coverslip and slide; take care not to break the coverslip. Apply epoxy to the edges of the slides.
NOTE: Do not add so much that epoxy fills in the drilled holes. - Place the flow chamber with the coverslip side facing downward in a humidified dark box to allow the epoxy to dry (~30 min).
NOTE: Add T50 buffer (Supplementary File 1) through the drilled holes to prevent the epoxy from covering the holes (10-15 µL) during the drying period. - Incubate each chamber lane with 500 nM Neutravidin (diluted in T50) by slowly applying ~40 µL to each lane.
- Incubate at RT for 2 min inside a humidified dark box. Wash with ~100 µL of T50 buffer per lane.
- Incubate each chamber lane with 20 nM biotinylated antibody11. The choice of antibody depends on the tag on the protein.
NOTE: If the primary antibody is not biotinylated, first incubate with the biotinylated secondary antibody for 30 min and then incubate with the primary antibody. - Incubate at RT for 30 min inside a humidified dark box. Wash with ~200 µL of T50 per lane.
NOTE: Ensure the lanes never dry out during the preparation process.
4. Single-molecule buffers
- Trolox buffer
NOTE: Trolox buffer is the starting buffer for making imaging buffer. Components of the buffer are dependent on the experiment and may vary depending on the protein of interest. The buffer used in the protocol described here includes salts (NaCl, KCl, CaCl2, MgCl2), buffering agent (HEPES), and Trolox (pH ~7.35).- Dissolve 9-10 mg of Trolox in 10 mL of single-molecule recording buffer (SRB, Supplementary File 1)
NOTE: Trolox makes the buffer slightly acidic. Adjust the pH at this stage using sodium hydroxide (NaOH) solution, 10 M (pH 7.35). The fine pH adjustment will be made after Trolox is fully dissolved; however, increasing the pH at this point increases the solubility of the Trolox. - Mix the solution at RT using a benchtop rocker for 4-8 h (wrapped in aluminum foil) to fully dissolve the Trolox.
- Check the pH and adjust if needed.
- Ensure the Trolox is fully dissolved. Sterilize the solution with a syringe filter and store at 4 °C.
NOTE: The buffer should be used after 2-10 days of aging. Trolox helps to suppress blinking and is commonly used in single-molecule studies17. The anti-blinking properties come from an oxidized derivative of Trolox18; hence, it is recommended to keep it at RT for at least a few hours to mature. Additionally, UV radiation of fresh Trolox solution speeds up the oxidation process and can be used to accelerate the "aging" of Trolox buffer18.
- Dissolve 9-10 mg of Trolox in 10 mL of single-molecule recording buffer (SRB, Supplementary File 1)
- Imaging buffer recipe: Mix Trolox buffer + detergent (~2 times the detergent's CMC value) + 4 mM protocatechuic acid (PCA).
NOTE: The detergent concentration is kept near CMC as high detergent concentrations may result in increased protein denaturation. For example, the following mixture can be used 955 µL Trolox + 5 µL 10% DDM + cholesterol (W%, 10:1) + 40 µL of 100 mM PCA stock solution. Here, PCA acts as an antioxidant agent and was previously used in smFRET studies19. DDM is non-ionic, is commonly used to solubilize membrane proteins20,21, and has been used in single-molecule studies. DDM is a good first choice detergent; however, we recommend testing multiple detergents and ensuring the results are consistent.
5. Microscope setup and smFRET data acquisition
- Turn on the computer and microscope. Turn on the lasers to warm up (532 nm for Cy3 excitation and 640 nm for Cy5 excitation).
NOTE: Here, an inverted microscope equipped with a 100x TIRF objective (N.A. 1.49), image splitter, and EMCCD camera was used. The setup is equipped with a four-line laser combiner, a dichroic mirror, a long pass emission filter, an emission dichroic filter, and a notch filter. - Turn on the EMCCD camera and open the camera software. Wait 20 min for the camera to reach −69 °C and stabilize.
- Mount the sample chamber on the microscope stage. Add the protein sample gradually to achieve ~400 molecules per field of view (step 2.4.5). Wash the chamber with 100 µL of the imaging buffer.
- Adjust the gain, acquisition rate, and laser powers such that single-molecule fluorescence signals are detected in both the donor and acceptor channels. Adjust the concentration of the proteins inside the sample chamber if needed.
NOTE: With more than 400 molecules in the field of view, distinguishing individual molecules becomes more difficult, and the background noise will be higher. - Excite the donor and acquire time traces until at least 80% of the donor molecules in the field of view are photobleached.
- At the end of the movie, turn on the 640 nm laser to directly excite the acceptor until some of the acceptor molecules photobleach, facilitating single-molecule from multimer discrimination.
- Move over to a different field of view and repeat the steps above to collect at least three movies (technical replicates) per condition.
NOTE: Use the lowest laser power possible while selecting a new region of interest (ROI) and focusing to minimize photobleaching. Pay attention to the stage drift during acquisition. If noticeable drift is observed after moving to a new ROI, wait for 3 min before starting acquisition.
6. Data analysis
- Donor and acceptor channel alignment (movie mapping)
- Record the fluorescent bead images in the donor and acceptor channels.
- Generate the mapping file using the bead data to correlate the donor and acceptor fluorescence from each molecule22,23.
NOTE: The emission signal from a single molecule is split into donor and acceptor signals by the emission dichroic filter inside the image splitter. The donor and acceptor images are projected on the camera side-by-side. To accurately associate the donor and acceptor intensities of a single molecule between the two areas, a mapping file is often generated using fluorescent bead samples. Using this mapping file, all the molecules that are detected in the donor and acceptor channels are mapped onto each other. The analysis then generates the time traces, which are the donor and acceptor intensities over time, for each molecule.
- Selection of single-molecule FRET traces (particle picking)
NOTE: Individual particle traces are examined and selected for downstream analysis using MATLAB. The exact selection criteria depend on the system. General guidelines on what constitutes a quality particle are outlined here. All the custom-modified codes are available on GitHub (https://github.com/vafabakhsh-lab).- Select the traces where the total intensity of the traces (donor + acceptor) is stable over time. Select the traces with anti-correlated changes in donor and acceptor intensities.
- Select the donor and acceptor molecules that show single-step photobleaching. Select the traces that are >5 s long.
NOTE: The background in each channel after bleaching should go to zero. The traces should not have many blinking events; this will increase the difficulty of analysis. - Calculate the FRET efficiency using the equation E = (IA− 0.088 × ID)/(ID + [IA− 0.088 × ID])24,25,26, where ID and IA are raw donor and acceptor intensities, respectively.
NOTE: The leakage of donor emission into the acceptor channel is determined using donor-only labeled sample excited using a 532 nm laser26. The leakage correction factor, 0.088, may differ for different setups depending on the filter sets used. It is important to note that quantitative and robust conversion of FRET efficiencies to absolute distances requires the correction of donor and acceptor intensities for multiple factors and has been extensively discussed before27.
- Identify the conformational state by Hidden Markov Modeling (HMM)
- Execute the vbFRET28 program in MATLAB and import the selected traces for a given condition. Set the constraints for the number of potential states and iterations to be executed.
NOTE: Based on the raw data from the representative results, it was hypothesized that there were up to four discrete FRET states occupied by the conformational sensor; thus, a range of one to four states was designated. Improvements in fitting were previously determined to negligibly increase with >25 iterations; thus, 25 iterations were used for fitting the representative data. - Analyze the smFRET traces and export the idealized traces and analysis session. Save the idealized traces to a separate folder for downstream analysis.
NOTE: The programs used to extract state transition and dwell-time data from idealized traces were made available in previously published work29. - Using MATLAB programs, extract state transitions and plot them as a heatmap with the X-coordinate indicating starting conformation and the Y-coordinate indicating ending conformation.
NOTE: Transitions are defined as changes in FRET value >0.1 in the representative results discussed here. The threshold for transitions is dependent on the hypothesized conformational states a protein of interest occupies (set transition threshold to be less than the difference between the closest FRET states), as well as the resolution allowed by the experimental setup. The examination of heatmaps for multiple conditions enables the identification of the most common conformational states a sensor transitions through and, thus, occupies. Four FRET states were identified for the representative results (FRET = 0.31, 0.51, 0.71, and 0.89). - Using MATLAB programs, extract the dwell-times for each identified conformational state. Designate a range of FRET values delineating each state and time resolution during data acquisition. FRET ranges are evenly divided by adjacent FRET states. Export the dwell-times for given treatment conditions.
NOTE: In most cases, dwell-time data can be estimated well by a single exponential decay function. This analysis can be performed in a data analysis and graphing software.
- Execute the vbFRET28 program in MATLAB and import the selected traces for a given condition. Set the constraints for the number of potential states and iterations to be executed.
- Gaussian fitting of smFRET population histograms to quantify state occupancy
- Import population FRET histograms for conditions of interest into the data analysis and graphing software for multiple peak fitting analysis.
- Indicate the number of peaks present (four peaks or states based on HMM analysis). Fitting was performed using multiple Gaussian distributions30 defined as
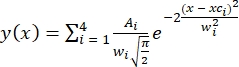 , where A is the peak area, xc is the peak center, and w is the peak width for each peak.
, where A is the peak area, xc is the peak center, and w is the peak width for each peak. - Constrain the fitting parameters as A > 0, xc = FRET ± 0.02, and 0.1 ≤w≤ 0.24. Four FRET peaks for individual population FRET histograms were fit simultaneously. Apply the defined constraints for the fittings of all conditions.
- Calculate the state of occupancy (percentage) as the area of peak of interest divided by the total area, defined as the sum of all peaks.
Results
Expression and fluorescent labeling of UAA-based FRET sensor
Herein, exemplary results of the insertion and fluorescent labeling of a UAA (AZP) within the CRD of mGluR2 (548UAA) are discussed11. As mentioned previously, to insert AZP into mGluR2, co-expression of the engineered translational machinery, which includes a modified tRNA synthetase and complementary tRNA (pIRE4-Azi), and mGluR2 containing an amber codon at position 548, created using mutagenesis, is necessary (
Discussion
GPCRs are proteins that operate on the cell membrane to initiate signal transduction. Many GPCRs consist of multiple domains, with signaling being dependent on the cooperative interaction between the domains. To modulate the properties of these membrane receptors, it is essential to understand the dynamic behavior of the multiple domains. Single-molecule fluorescence resonance energy transfer (smFRET) is a fluorescence technique that enables the measurement of protein conformation and dynamics in real time
Disclosures
The authors declare no competing interests.
Acknowledgements
We thank members of the Reza Vafabakhsh lab for discussions. This work was supported by the National Institutes of Health grant R01GM140272 (to R.V.), by The Searle Leadership Fund for the Life Sciences at Northwestern University, and by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (to R.V.). B.W.L. was supported by the National Institute of General Medical Sciences (NIGMS) Training Grant T32GM-008061.
Materials
| Name | Company | Catalog Number | Comments |
| (+)-Sodium L-Ascorbate | Sigma Aldrich | Cat # 11140-250G | |
| 4-azido-L-phenylalanine | Chem-Impex International | Cat # 06162 | |
| 548UAA | Liauw et al. 2021 | Transfected construct | |
| Acetic Acid | Fisher Chemical | 64-19-7 | |
| Acetone | Fisher Chemical | 67-64-1 | |
| Adobe Illustrator (2022) | https://www.adobe.com/ | RRID:SCR_010279 | Software, algorithm |
| Aminoguanidine (hydrochloride) | Cayman Chemical | 81530 | |
| Aminosilane | Aldrich | 919-30-2 | |
| Bath Sonicator 2.8 L | Fisher Scientific | Ultrasonic Bath 2.8 L | |
| Biotin-PEG | Laysan Bio Inc | Item# Biotin-PEG-SVA-5000-100mg | |
| BTTES | Click Chemistry Tools | 1237-500 | |
| Copper (II) sulfate | Sigma Aldrich | Cat # 451657-10G | |
| Cover slip | VWR | 16004-306 | Sample chamber |
| Cy3 Alkyne | Click Chemistry Tools | TA117-5 | |
| Cy5 Alkyne | Click Chemistry Tools | TA116-5 | |
| DDM | Anatrace | Part# D310 1 GM | Detergent |
| DDM-CHS (10:1) | Anatrace | Part# D310-CH210 1 ML | Detergent with cholecterol |
| Defined Fetal Bovine Serum | Thermo Fisher Scientific | SH30070.03 | |
| Di01-R405/488/561/635 | Semrock | Notch filter | |
| DMEM | Corning | 10-013-CV | |
| EMCCD | Andor | DU-897U | Camera |
| ET542lp | Chroma | Long pass emission filter | |
| FF640-FDi01 | Semrock | Emission dichroic filter | |
| FLAG-tag antibody | Genscript | A01429 | |
| Fluorescent bead | Invitrogen T7279 | TetraSpeck microspheres | Spherical bead |
| Glass slides | Fisherfinest | 12-544-4 | sample chamber |
| Glutamate | Sigma Aldrich | Cat # 6106-04-3 | |
| HEK 293T | Sigma Aldrich | Cat # 12022001 | Cell line |
| HEPES | FisherBioReagents | 7365-45-9 | |
| Image splitter | OptoSplit II | ||
| KOH | Fluka | 1310-58-3 | |
| Laser | Oxxius | 4-line laser combiner | |
| Lipofectamine 3000 Transfection Reagent | Thermo Fisher Scientific | L3000015 | Transfection Reagent |
| Methanol | Fisher Chemical | 67-56-1 | |
| Microscope | Olympus | Olympus IX83 | |
| Milli-Q water | Barnstead | Water Deionizer | |
| m-PEG | Laysan Bio Inc | Item# MPEG-SIL-5000-1g | |
| NF03-405/488/532/635 | Semrock | Dichroic mirror | |
| OptiMEM | Thermo Fisher Scientific | 51985091 | Reduced Serum Medium |
| OptiMEM/Reduced serum medium | Thermo Fisher Scientific | ||
| OriginPro (2020b) | https://www.originlab.com/ | RRID:SCR_014212 | Data analysis and graphing software |
| Penicillin-Streptomycin | Gibco | 15140-122 | |
| pIRE4-Azi | Addgene | Plasmid # 105829 | Transfected construct |
| Poly-L-lysine hydrobromide | Sigma Aldrich | Cat # P2636 | |
| Protocatechuic acid (PCA) | HWI group | 99-50-3 | |
| smCamera (Version 1.0) | http://ha.med.jhmi.edu/resources/ | Camera software | |
| Sodium bicarbonate | FisherBioReagents | 144-55-8 | |
| Sodium hydroxide (NaOH) | Sigma | 1310-73-2 | |
| Syringe filter | Whatman UNIFLO | Cat#9914-2502 | Liquid filtration |
| Trolox | Sigma | 53188-07 |
References
- Smock, R. G., Gierasch, L. M. Sending signals dynamically. Science. 324 (5924), 198-203 (2009).
- Changeux, J. P., Christopoulos, A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell. 166 (5), 1084-1102 (2016).
- Tang, X. -. l., Wang, Y., Li, D. -. l., Luo, J., Liu, M. -. Y. Orphan G protein-coupled receptors (GPCRs): biological functions and potential drug targets. Acta Pharmacologica Sinica. 33 (3), 363-371 (2012).
- Chung, K. Y., et al. Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. Nature. 477 (7366), 611-615 (2011).
- Vafabakhsh, R., Levitz, J., Isacoff, E. Y. Conformational dynamics of a class C G-protein-coupled receptor. Nature. 524 (7566), 497-501 (2015).
- Niswender, C. M., Conn, P. J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annual Review of Pharmacology and Toxicology. 50, 295-322 (2010).
- Pin, J. P., Bettler, B. Organization and functions of mGlu and GABA(B) receptor complexes. Nature. 540 (7631), 60-68 (2016).
- Kniazeff, J., et al. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nature Structural & Molecular Biology. 11 (8), 706-713 (2004).
- Ha, T. Single-molecule fluorescence resonance energy transfer. Methods. 25 (1), 78-86 (2001).
- Schuler, B., Eaton, W. A. Protein folding studied by single-molecule FRET. Current Opinion in Structural Biology. 18 (1), 16-26 (2008).
- Liauw, B. W. -. H., Afsari, H. S., Vafabakhsh, R. Conformational rearrangement during activation of a metabotropic glutamate receptor. Nature Chemical Biology. 17 (3), 291-297 (2021).
- Noren, C. J., Anthonycahill, S. J., Griffith, M. C., Schultz, P. G. A general method for site-specific incorporation of unnatural amino acids into proteins. Science. 244 (4901), 182-188 (1989).
- Presolski, S. I., Hong, V. P., Finn, M. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Current Protocols in Chemical Biology. 3 (4), 153-162 (2011).
- Huber, T., Naganathan, S., Tian, H., Ye, S. X., Sakmar, T. P. Unnatural amino acid mutagenesis of GPCRs using amber codon suppression and bioorthogonal labeling. G Protein Coupled Receptors: Structure. 520, 281-305 (2013).
- Serfling, R., Coin, I., Pecoraro, V. Chapter Four - Incorporation of Unnatural Amino Acids into Proteins Expressed in Mammalian Cells. Methods in Enzymology. 580, 89-107 (2016).
- Chandradoss, S. D., et al. Surface passivation for single-molecule protein studies. Journal of Visualized Experiments. (86), e50549 (2014).
- Rasnik, I., McKinney, S. A., Ha, T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nature Methods. 3 (11), 891-893 (2006).
- Cordes, T., Vogelsang, J., Tinnefeld, P. On the mechanism of Trolox as antiblinking and antibleaching reagent. Journal of the American Chemical Society. 131 (14), 5018-5019 (2009).
- Aitken, C. E., Marshall, R. A., Puglisi, J. D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophysical Journal. 94 (5), 1826-1835 (2008).
- Lee, S., et al. How do short chain nonionic detergents destabilize G-protein-coupled receptors. Journal of the American Chemical Society. 138 (47), 15425-15433 (2016).
- Cao, A. -. M., et al. Allosteric modulators enhance agonist efficacy by increasing the residence time of a GPCR in the active state. Nature Communications. 12 (1), 1-13 (2021).
- Mancebo, A., Mehra, D., Banerjee, C., Kim, D. -. H., Puchner, E. M. Efficient cross-correlation filtering of one-and two-color single molecule localization microscopy data. Frontiers in Bioinformatics. 1, 739769 (2021).
- Mehra, D., Adhikari, S., Banerjee, C., Puchner, E. M. Characterizing locus specific chromatin structure and dynamics with correlative conventional and super-resolution imaging in living cells. Nucleic Acids Research. , (2022).
- Chen, H., Puhl, H. L., Koushik, S. V., Vogel, S. S., Ikeda, S. R. Measurement of FRET efficiency and ratio of donor to acceptor concentration in living cells. Biophysical Journal. 91 (5), 39-41 (2006).
- Gopich, I. V., Szabo, A. FRET efficiency distributions of multistate single molecules. The Journal of Physical Chemistry B. 114 (46), 15221-15226 (2010).
- Roy, R., Hohng, S., Ha, T. A practical guide to single-molecule FRET. Nature Methods. 5 (6), 507-516 (2008).
- Hellenkamp, B., et al. Precision and accuracy of single-molecule FRET measurements-A multi-laboratory benchmark study. Nature Methods. 15 (9), 669-676 (2018).
- Bronson, J. E., Fei, J., Hofman, J. M., Gonzalez, R. L., Wiggins, C. H. Learning rates and states from biophysical time series: A Bayesian approach to model selection and single-molecule FRET data. Biophysical Journal. 97 (12), 3196-3205 (2009).
- Zhang, J., et al. Specific structural elements of the T-box riboswitch drive the two-step binding of the tRNA ligand. Elife. 7, 39518 (2018).
- Goodman, N. R. Statistical analysis based on a certain multivariate complex Gaussian distribution (an introduction). The Annals of Mathematical Statistics. 34 (1), 152-177 (1963).
- Brown, R. B., Audet, J. Current techniques for single-cell lysis. Journal of the Royal Society Interface. 5, 131-138 (2008).
- Schamber, M. R., Vafabakhsh, R. Mechanism of sensitivity modulation in the calcium-sensing receptor via electrostatic tuning. Nature Communications. 13 (1), 2194 (2022).
- Jain, A., Liu, R., Xiang, Y. K., Ha, T. Single-molecule pull-down for studying protein interactions. Nature Protocols. 7 (3), 445-452 (2012).
- Huang, S. K., et al. Delineating the conformational landscape of the adenosine A(2A) receptor during G protein coupling. Cell. 184 (7), 1884-1894 (2021).
- Wingler, L. M., et al. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell. 176 (3), 468-478 (2019).
- Gordon, C. G., et al. Reactivity of biarylazacyclooctynones in copper-free click chemistry. Journal of the American Chemical Society. 134 (22), 9199-9208 (2012).
- Kim, E., Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chemical Science. 10 (34), 7835-7851 (2019).
- Pickens, C. J., Johnson, S. N., Pressnall, M. M., Leon, M. A., Berkland, C. J. Practical considerations, challenges, and limitations of bioconjugation via azide-alkyne cycloaddition. Bioconjugate Chemistry. 29 (3), 686-701 (2018).
- Geng, Y., et al. Structural mechanism of ligand activation in human calcium-sensing receptor. Elife. 5, 13662 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved