Method Article
In Vitro Selection of Engineered Transcriptional Repressors for Targeted Epigenetic Silencing
In This Article
Summary
Here, we present a protocol for the in vitro selection of engineered transcriptional repressors (ETRs) with high, long-term, stable, on-target silencing efficiency and low genome-wide, off-target activity. This workflow allows for reducing an initial, complex repertoire of candidate ETRs to a short list, suitable for further evaluation in therapeutically relevant settings.
Abstract
Gene inactivation is instrumental to study gene function and represents a promising strategy for the treatment of a broad range of diseases. Among traditional technologies, RNA interference suffers from partial target abrogation and the requirement for life-long treatments. In contrast, artificial nucleases can impose stable gene inactivation through induction of a DNA double strand break (DSB), but recent studies are questioning the safety of this approach. Targeted epigenetic editing via engineered transcriptional repressors (ETRs) may represent a solution, as a single administration of specific ETR combinations can lead to durable silencing without inducing DNA breaks.
ETRs are proteins containing a programmable DNA-binding domain (DBD) and effectors from naturally occurring transcriptional repressors. Specifically, a combination of three ETRs equipped with the KRAB domain of human ZNF10, the catalytic domain of human DNMT3A and human DNMT3L, was shown to induce heritable repressive epigenetic states on the ETR-target gene. The hit-and-run nature of this platform, the lack of impact on the DNA sequence of the target, and the possibility to revert to the repressive state by DNA demethylation on demand, make epigenetic silencing a game-changing tool. A critical step is the identification of the proper ETRs' position on the target gene to maximize on-target and minimize off-target silencing. Performing this step in the final ex vivo or in vivo preclinical setting can be cumbersome.
Taking the CRISPR/catalytically dead Cas9 system as a paradigmatic DBD for ETRs, this paper describes a protocol consisting of the in vitro screen of guide RNAs (gRNAs) coupled to the triple-ETR combination for efficient on-target silencing, followed by evaluation of the genome-wide specificity profile of top hits. This allows for reduction of the initial repertoire of candidate gRNAs to a short list of promising ones, whose complexity is suitable for their final evaluation in the therapeutically relevant setting of interest.
Introduction
Gene inactivation has traditionally played a key role to study gene function in both cellular and animal models. Furthermore, in the last two decades, with the rise of gene therapy, it has been proposed as a potentially game-changing approach to treat diseases caused by gain-of-function mutations1, infectious diseases2, or pathologies in which silencing of one gene may compensate for an inherited defect in another one3. Finally, genetic inactivation of key regulators of cell fitness and functional control has been proposed to enhance the efficiency of cell products for cancer immunotherapy4 and regenerative medicine5.
Among the different technologies to accomplish gene inactivation, one of the most promising is targeted epigenetic silencing6,7. At the core of this technology are the so-called engineered transcriptional repressors (ETRs), chimeric proteins consisting of a programmable DNA-binding domain (DBD) and an effector domain (ED) with epigenetic repressive function. Zinc finger proteins (ZFPs)8, transcription activator-like effectors (TALEs)9, or CRISPR/dCas910-based DBDs can be designed to selectively tether the ED to the promoter/enhancer sequence of the target gene to be silenced. Once there, the ED of the ETR performs its silencing activity by imposing heterochromatin-inducing repressive epigenetic marks such as histone modifications (H3K911,12 or H3K2713 methylation, H3 or H4 deacetylation14) and CpG DNA methylation15, according to the repressive domain used.
In particular, inspired by the molecular processes of permanent transcriptional repression of endogenous retroviruses occurring in the pre-implantation embryo16, a combination of three ETRs has been generated to exploit the following EDs: i) the Krüppel-associated box (KRAB) domain of human ZNF10; ii) the catalytic domain of human de novo DNA methyltransferase 3A (DNMT3A); and iii) the full-length human DNA methyltransferase 3-like (DNMT3L). KRAB is a conserved repressive domain shared by several ZFPs in higher vertebrates17,18, whose silencing activity is mainly based on its ability to recruit KAP119-a scaffold protein that then interacts with several other heterochromatin inducers20-comprising the nucleosome remodeling and deacetylation (NuRD) complex21, the H3K9 histone methyltransferase SETDB122, and the H3K9 methylation reader HP123,24, among others.
DNMT3A actively transfers methyl groups on the DNA at CpG sequences25. The catalytic activity of DNMT3A is enhanced by its physical association with DNMT3L, an embryo- and germ cell-restricted paralog of DNMT3A lacking the catalytic domain responsible for methyl group transfer26,27. DNA methylation at CpG-rich regions-referred to as CpG islands (CGIs)-embedded in the promoter/enhancer elements of mammalian genes is usually associated with transcription silencing28. Importantly, once deposited, CpG methylation can be stably inherited throughout mitosis by a UHRF1-DNMT1-based molecular complex29.
Stable overexpression of the ETRs in the target cell can be problematic, likely because of the increasing risks of off-target activity and squelching of endogenous interactors from their physiological target sites over time. However, transient expression of single-ETR moieties can fail to induce long-term silencing with high efficiency30, hampering their therapeutic application. Therefore, a seminal breakthrough in the field was the evidence that the combination of the three KRAB-, DNMT3A-, and DNMT3L-based ETRs can synergize and, even when only transiently co-delivered, impose on the promoter sequence of the target gene H3K9 and CpG methylation. These are then read and propagated by the cell throughout mitosis, leading to heritable silencing in multiple human and murine cell lines, as well as ex vivo cultured primary cells30.
Of note, the epigenetic silencing imposed by the ETRs can be reverted on demand by targeted (e.g., recruitment of the CRISPR/dCas9-based TET1 DNA demethylase on the silenced locus) or pharmacological (administration of the DNA methyltransferase inhibitor 5-Aza) DNA demethylation30, a potential antidote in case of ETR-related adverse events. All-in-one ETRs bearing the three KRAB-, DNMT3A-, and DNMT3L-based EDs were also described, showing significant silencing efficiencies in cell lines31,32 against the large majority of protein-coding genes. Furthermore, several studies employing the ETRs reported a high safety profile, with no major off-target activity in terms of de novo CpG methylation or alteration of chromatin accessibility30,31,32. However, a dedicated analysis of the specificity profile of ETRs equipped with a newly designed DBD is recommended before clinical applications.
From a clinical perspective, targeted epigenetic silencing may provide critical advantages to both RNA interference (RNAi)-based knockdown33 and artificial nuclease-based gene disruption8. In contrast to RNAi, targeted epigenetic silencing may induce full abrogation of its target per cell and does not require periodic treatment to ensure long-term silencing; in contrast to gene disruption, it leaves the DNA sequence unaltered, avoiding the generation of DNA double-strand breaks (DSBs). DSBs can then induce apoptosis and cell cycle arrest, potentially leading to a selection against cells with a functional p53 pathway34,35 and, especially in multiplex gene editing settings, chromosomal rearrangements35. Furthermore, by relaying the irreversible mosaic outcome of non-homologous-end-joining-mediated DNA DSB repair36, gene disruption cannot avoid in-frame repair of the target into functional coding sequences as one of the final outcomes and, in contrast to epigenetic silencing, cannot be erased on demand.
Finally, epigenetic silencing holds the potential to broaden the range of targetable genetic elements to classes fully or at least partially refractory to RNAi and gene disruption, such as non-transcribed regulatory elements and non-coding RNAs30,32. The first critical step for any targeted epigenetic silencing application is to design a panel of ETRs covering the different regulatory sequences of the target gene and identify the best-performing ones. The number of ETRs to be tested can be crucial, considering the increasing portion of the genome that can be targeted by the programmable DNA binding technologies constantly under development37. Performing the screen of the ETRs directly on the cell type in which to therapeutically silence the target gene would represent the most relevant option. However, high-throughput screens can be technically cumbersome in primary cells due to their limited survival in culture and their often suboptimal engineering capacity. Large-scale screens can be even more unfeasible in vivo.
A more practical alternative consists of performing an initial screening of a large panel of ETRs in easily engineerable cell lines at first, and then only validating the most promising ones in the therapeutically relevant cell type. A parallel issue is the selection of an appropriate readout to measure the silencing efficiency of the ETRs. Directly assessing the transcript or protein levels of the target gene by RT-qPCR, western blot, or ELISA can be costly and time-consuming and may lack sufficient sensibility, thus limiting their application at high-throughput scales. The generation of ad hoc engineered reporter cell lines in which a fluorophore is placed under the transcriptional control of the regulatory sequences of the target gene allows exploitation of the flow cytometry-based approach to read epigenetic silencing at the single-cell level and at high-throughput pace.
Following these general considerations, this paper describes a protocol consisting of the in vitro arrayed screen of ETRs for on-target silencing efficiency, followed by evaluation of the genome-wide off-target activity of the top hits. This workflow allows for reduction of the initial repertoire of candidate ETRs to a short list of promising ones, whose complexity is suitable for their final evaluation in the therapeutically relevant cell type of interest.
Among the different programmable DBDs that can be exploited to generate ETRs, this protocol will focus on the CRISPR/dCas9-based technology, because of the ease of designing gRNAs spanning the target gene promoter at a high-throughput scale. However, the same conceptual workflow described below can be adopted to evaluate the efficiency and specificity of ETRs equipped with other DBDs.
Protocol
1. Engineering a fluorescence-based reporter cell line to monitor the transcriptional activity of the target gene by flow cytometry
- Identify cell lines expressing the target gene to be silenced. Browse the target gene to be silenced in the Human Protein Atlas38 and navigate through its "Cell line" section to identify those lines representative of the somatic tissue of interest (e.g., a hepatic cell line if the final targets are liver hepatocytes). Alternatively, interrogate a publicly available RNA sequencing (RNA-Seq) database (e.g., NCBI GEO).
- Among the candidates, prioritize cell lines for which efficient transient gene delivery protocols-instrumental for ETR delivery-are available.
NOTE: Among the different modalities, nucleofection represents one of the best options, as it ensures high transfection efficiencies. Here, human erythroleukemia K-562 cells have been chosen to generate a cell line reporting the transcriptional activity of the beta-2-microglobulin (B2M) gene (hereafter referred as to the B2MTdTomato K-562 cells). - Further prioritize the candidates to avoid cell lines in which the target gene is essential for cell viability, as this impairs the maintenance of cells with stable silencing of the target gene in culture. If not previously reported, to have a sense of the essentiality of the target gene in the cell type of choice, generate a genetic disruption control by transfecting the cells with Cas9 nuclease and a gRNA targeting one of the first coding exons of the gene.
NOTE: Genetic disruption is a stable event by definition; counterselection of disrupted cells over time indicates that the target gene is essential for the physiology of the selected cell.- Identify the target splicing isoform preferentially used in the selected cell line (the isoform NM_004048.4 of the B2M gene is targeted in this protocol).
- Identify a gRNA that can cut effectively and specifically in the first coding exon of the target isoform (e.g., Chopchop (http://chopchop.cbu.uib.no/)39, which is a valid and user-friendly online gRNA selection tool).
- For K-562 cells, transfect 1 µg of spCas9-encoding plasmid (hCas9; see Table of Materials) and 250 ng of gRNA-encoding plasmid (phU6-gRNA; see Table of Materials) per 5 × 105 cells through nucleofection (according to the manufacturer's instructions).
- Culture the cells (K-562 cells at 37 °C under 5% CO2 in RPMI-1640 supplemented with 10% fetal bovine serum [FBS], L-glutamine, and penicillin/streptomycin [100 U/mL]) and monitor the levels of gene disruption over time by exploiting a mutation detection kit (follow the manufacturer's instructions).
- Clone a donor template for homologous recombination-mediated integration of a fluorescent reporter under the transcriptional control of the target gene of interest (Figure 1).
- Identify the region of the target gene to integrate the fluorophore expression cassette.
NOTE: Avoid targeting transcriptionally relevant elements, such as CpG islands and regions enriched for H3K27 acetylation (marker of active promoters and enhancers). Altering these regulatory elements (potentially important to be targeted by the ETRs to instruct epigenetic silencing) makes the reporter cell line less predictive of the physiological regulation of the target gene.- If the target gene does not encode for a secreted protein, fuse the reporter to the last codon of the target gene through a 2A self-cleaving peptide to maintain the functionality of the target gene.
- If the target gene does encode for a secreted protein, to avoid potential secretion of the reporter, place the reporter in an intronic region of the target gene. Force integration of the reporter in the spliced transcript through a splice acceptor site (SA) and subsequent translation through an internal ribosome entry sequence (IRES) impairs the functionality of the target gene.
- Use Chopchop to select gRNA cutting in the target region (here, a gRNA is selected to target the sequence 5′-AGGCTACTAGCCCCATCAAGAGG-3′ of the first intron of the B2M gene).
- Design a donor template for the gRNA cut site consisting of: i) a left homology arm (n base pairs [bp] matching the region just upstream of the gRNA cut site); ii) a promoter-free transgene expression cassette (in the case here shown, an SA-3X Stop Codon-IRES-tdTomato-BGH poly(A) favoring splicing with the first intron of the B2M gene); and iii) a right homology arm (n bp matching the region downstream of the gRNA cut site).
NOTE: The length of the homology arms necessary to effectively induce homologous recombination can vary between different cell types (100-500 bp is a proper range for K-562 cells).
- Identify the region of the target gene to integrate the fluorophore expression cassette.
- Deliver the CRISPR/Cas9 nuclease system and the donor template inside the target cell line. For K-562 cells, transfect 1 µg of spCas9-encoding plasmid (hCas9; see Table of Materials), 250 ng of gRNA-encoding plasmid (phU6-gRNA; see Table of Materials), and 1 µg of donor template-encoding plasmid per 5 × 105 cells through nucleofection (according to the manufacturer's instructions).
- Culture the cells for at least 14 days (for K-562 cells) and monitor the expression levels of the fluorescent reporter over time by using a flow cytometer (activate the phycoerythrin (PE) channel to measure the fluorescence intensity of the tdTomato reporter and follow the manufacturer's instructions to perform flow cytometry).
NOTE: The donor template-especially if plasmid-based-can contain cryptic promoter sequences, leading to the expression of the fluorescent reporter from non-integrated donor copies. Culturing the cells allows for dilution of these non-integrated copies by cell division and finally maintaining the expression of the reporter only from the donor copies integrated in the target genome. - Clone reporter-positive cells through fluorescence activated cell sorting (FACS) at a single-cell level. For this protocol, activate the PE channel to measure the fluorescence intensity of the tdTomato reporter and follow the manufacturer's instructions to sort single tdTomato-positive K-562 cells per well of a 96-well plate.
- Upon cell expansion in culture (typically 20-30 days for K-562 cells), screen reporter-positive clones by PCR to select one bearing a bi-allelic integration of the reporter cassette inside the target locus.
NOTE: This maximizes reporter expression and facilitates further resolution between reporter-expressing and reporter-silenced cells by flow cytometry upon ETR treatment.- Extract genomic DNA from 1 × 105 cells per reporter-positive clone using a DNA extraction kit (following the manufacturer's instructions).
- Amplify the B2M target region with forward (5'-GTATTTGCTGGTTATGTTAG-3') and reverse (5'-AATGGTTGAGTTGGAC-3') primers following the instructions of the PCR amplification kit. The annealing temperature for this pair of primers is 47.7 °C with Taq-based DNA polymerases and 0.5 µM primer concentrations.
- Analyze the PCR product using 1% agarose gel electrophoresis (following the manufacturer's instructions). Screen for clones showing the band related to integration of the tdTomato in the B2M target locus (3,413 bp), without the band related to the wild-type target locus (1,027 bp).
2. Designing gRNAs for CRISPR/dCas 9-based epigenetic silencing of the target gene
- Browse the target gene in the UCSC genome browser40 and extract the nucleotide sequence of regions potentially regulating its transcriptional activity, such as CpG islands and sites enriched for H3K27 acetylation (marker of active promoters and enhancers).
NOTE: According to a recent study, the best targeting region is a 1 kilobase (kb) window centered on the transcription start site of the target gene32. - Paste the selected sequences in the Chopchop online tool and select repression as the purpose of the gRNAs to be retrieved. Wait for Chopchop to provide a list of gRNAs mapped on the genetic sequence of interest and listed according to a score considering both the number of off-target matches and the predicted on-target efficiency (Figure 2).
- Select at least 10 gRNAs per target sequence. If possible, try to select gRNAs spanning throughout the whole region to be interrogated, with no full matches with other intragenic sequences throughout the genome.
3. Arrayed transient delivery of CRISPR/dCas 9-based ETRs in the reporter cell line
- Among the transgene delivery systems previously reported for the target cell line, choose those allowing only transient transgene expression, maximizing delivery efficiency, and minimizing cell manipulation-related toxicity. In the case of K-562 cells, both plasmid and mRNA nucleofection are highly recommended, with plasmid production representing a technically easier and cheaper alternative.
- Clone both the gRNAs selected in section 2 and CRISPR/dCas9-based ETRs in the transgene delivery system of choice. See the following steps for a protocol for cloning the ETRs in plasmid DNAs.
NOTE: Plasmids separately encoding for dCas9:KRAB, dCas9:DNMT3A, and dCas9:DNMT3L30 are not available on Addgene. They were cloned by replacing the VP160 trans-activator from the plasmid pAC154-dual-dCas9VP160-sgExpression41 (see Table of Materials) with either the KRAB, DNMT3A, or DNMT3L domain-coding sequence30. A plasmid encoding for an all-in-one ETR, termed CRISPRoff-v2.132, is available (see Table of Materials).- Transform plasmids encoding for the ETRs in chemically competent E. coli cells (following the manufacturer's instructions). Screen the colonies for the presence of the ETR-bearing plasmid by restriction enzyme digestion and Sanger sequencing, and finally choose one of the positive colonies for plasmid DNA Midiprep production (following the manufacturer's instructions).
- Clone the gRNAs inside the phU6-gRNA backbone (Figure 3).
- Using molecular biology design software, append a 5'-CACCG-3' sequence upstream of the protospacer (the first variable 20 nucleotides [nt] of the selected gRNA) to generate a 25 nt-long oligo in silico, referred to as SGfw.
- Similarly, append a 5'-AAAC-3' sequence upstream of the reverse complement of the protospacer of the selected gRNA and a 5'-C-3' downstream of it to generate a 25 nt-long oligo referred as SGrv.
- Order both the SGfw and SGrv sequences as salt-free single-stranded DNA oligos, resuspended in water at 100 µM.
- Add 1 µL of each oligo to 2 µL of annealing buffer (10 mM Tris [pH 7.5-8.0], 50 mM NaCl, 1 mM EDTA) and 16 µL of water.
- Perform oligo annealing by placing the solution in a thermocycler programmed to start at 95 °C for 10 min. Then, gradually cool to 25 °C over 45 min.
- Dilute 1 µL of the annealed oligos with 99 µL of nuclease-free water, and then ligate 1 µL of this dilution with 50 ng of phU6-gRNA plasmid previously digested with the BsaI restriction enzyme (follow the instructions of BsaI and ligase kit vendors for the digestion and ligation procedure).
- Transform 20 µL of chemically competent E. coli cells with 2 µL of the ligation product (follow the manufacturer's instructions for the transformation procedure).
- Pick multiple colonies for plasmid DNA Miniprep production (follow the instructions of the vendor) and control the successful cloning of the protospacer by Sanger sequencing with the following primer matching to the U6 promoter 5'-GAGGGCCTATTTCCCATGATT-3'.
- Choose one of the positive colonies for plasmid DNA Midiprep production (following the manufacturer's instructions).
- Deliver the selected gRNAs and CRISPR/dCas9-based ETRs in the reporter cell line in array (one specific gRNA per condition) (Figure 3).
NOTE: As a representative case, the workflow for the nucleofection of plasmids encoding for dCas9:KRAB, dCas9:DNMT3A, dCas9:DNMT3L, and gRNAs targeting the B2M CpG island in B2MTdTomato K-562 cells is shown in the following steps.- Prepare separate tubes containing 500 ng of each of the dCas9:KRAB-, dCas9:DNMT3A-, and dCas9:DNMT3L-encoding plasmids, but differing for the gRNA to be tested (125 ng of a gRNA-encoding plasmid per tube). Include a gRNA- and ETR-free nucleofection condition as mock-treated sample. Perform the screen with at least three technical replicates per sample.
- Pellet 5 × 105 B2MTdTomato K-562 cells per tube and nucleofect them with the plasmid mix (following the manufacturer's instructions).
- Resuspend the cells in 200 µL of previously warmed RPMI-1640 mammalian cell culture media and place them back in the incubator.
4. Analyzing the transcriptional activity of the target gene over time
- Use flow cytometry to measure the percentage of silenced cells at different time points after delivery of the ETRs (Figure 4). Use wild-type (WT) cells-not bearing the fluorophore-coding sequence-to set the threshold of reporter-negative cells. Use the mock-treated sample to set the gate for reporter-positive cells.
NOTE: As suggested in step 1.3, include a genetic disruption control in the experiment. This can be useful to both monitor the CRISPR delivery efficiency and the fitness of cells deprived of the target gene; the loss of both transcriptional silencing and genetic disruption over time can be ascribed to the essentiality of the target gene in the cell type of choice. Include both short- (day 3, day 7, day 10) and long-term (day 21, day 35) time points to get an indication of both acute and long-term efficiency of silencing. - Identify the top three gRNAs in terms of long-term silencing efficiency. Use FACS to select the reporter-negative subpopulation stably maintained in those samples. Also, perform FACS of the bulk mock-treated samples to keep them under the same treatment as the test samples to allow proper comparison in the subsequent analyses.
5. Evaluating the specificity of the ETR treatment by RNA-seq and methylated DNA immunoprecipitation (MeDIP)-seq
- Use RNA-seq to evaluate any eventual genome-wide transcriptional deregulation upon ETR delivery.
- For both the reporter-silenced subpopulation of the samples treated with the three top-performing gRNAs and mock-treated cells, extract RNA using commercially available kits. Assess the quality and concentration of the RNA by using commercially available kits.
- Perform RNA fragmentation, retrotranscription, and library preparation using commercially available kits for the preparation of RNA-seq libraries (following the manufacturer's instructions).
- Perform library quantification and quality control using quality control instruments compatible with next-generation sequencing and digital electrophoresis.
- Sequence libraries on a next generation sequencer following the manufacturer's instruction, with a 100 bp paired-end protocol and aiming at an average of 45 M reads/sample.
- Align read tags to the appropriate reference genome and quantify transcript expression. Perform alignment on the tdTomato sequence and quantify it separately.
NOTE: Here, STAR aligner (v 2.3.0)43, with default parameters, coupled to Rsubread package44 is used. - Perform analysis of the RNA-seq data according to published best practices45.
NOTE: The R/Bioconductor package edgeR46 is used here, applying a filter of at least one count per million (cpm) in at least three samples to discard low-expressed genes. Alternatively, the filterByExpr function in edgeR can be used. - Evaluate differential gene expression using a negative binomial generalized log-linear model implemented edgeR (function glmFit)47. Set a threshold of 0.01 on adjusted p values (Benjamini-Hochberg [BH] correction) to retain differentially regulated genes.
- Evaluate any eventual off-target CpG methylation activity of the ETRs by MeDIP-seq.
- For both the reporter-silenced subpopulation of the samples treated with the top three gRNAs and mock-treated cells, extract genomic DNA using commercially available kits (following the manufacturers' instructions).
- Sonicate 500 ng of genomic DNA using an ultrasonicator and the following parameters: Duty: 20 %; PIP: 175; Cycles per Burst: 200; Time: 40 s.
- Prepare sequencing libraries with the commercially available kits for MeDIP-seq (following the manufacturers' instructions).
- After the adaptor ligation step, quantify libraries by fluorometric assay and check the ligation efficiency by qPCR using commercially available library quantification kits (following the manufacturers' instructions).
- Obtain library pools by mixing randomly-selected libraries to reduce technical biases. Use an equal library amount (ng) for each library to balance the pools. To each pool, add the methylated and unmethylated spike-in control DNA provided in the kit. For control purposes, keep a 10% volume of the library, labeled as "input" and not immunoprecipitated. Perform immunoprecipitation on the remaining 90% of the library using the monoclonal antibody directed against 5-methylcytosine provided in the MeDIP-seq kit.
- Purify enriched and input libraries using the kits for purification of the 5-methylcytosine immunoprecipitation product, following the manufacturers' instructions.
- Evaluate the enrichment efficiency by performing quantitative real-time PCR on the internal spike in controls using primers provided with the kit. For each immunoprecipitation (IP), compute enrichment specificity from the recovery of methylated and unmethylated DNA, using the cycle threshold (Ct) values of MeDIP and input fractions obtained from the qPCR reaction (see equations 1and 2):
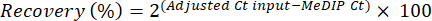 (1)
(1)
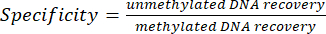 (2)
(2)
NOTE: Consider IP libraries to be successful if the specificity values are ≥0.95. - Amplify the libraries using MeDIP-seq library preparation kits, following the manufacturers' instructions, and perform quantification and library size distribution analysis of the product.
- Perform library sequencing on next generation sequencers. Use paired-end sequencing, with a read length of 100 bp, aiming at an average of 30 M reads/sample.
- Align the sequencing read tags to the appropriate reference genome (e.g., hg38) using bwa (v 0.7.5 or higher)48 and then identify peaks using MACS (v 2.0.10 or higher)49, allowing for the identification of broad peaks (-slocal = 0,-llocal = 500000).
- Create a common set of regions from different samples using BEDTools' multiintersection tool50, enabling the clustering option.
- Calculate the per-sample coverage over the final region list using BEDTools' multicov, discarding duplicated reads.
- Perform analysis of the matrix count using edgeR. Apply a filter of at least one count per million (cpm) in at least three samples to discard low-enriched regions.
NOTE: Alternatively, the filterByExpr function in edgeR could be used. - Identify differential methylation by adopting the generalized log-linear model implemented in edgeR (function glmFit) and normalizing using conditional quantile normalization51 to correct for region-wise GC-content. Select differentially methylated regions by applying a threshold of 0.01 on BH adjusted p values. Perform the analysis of repeated sequences as follows.
NOTE: Refer to the edgeR user guide for the full list of options and parameters (https://bioconductor.org/packages/release/bioc/html/edgeR.html).- Filter MeDIP-seq results for a nominal p value <0.01 and create two sets of regions: select regions having logFC >1 in the first set and regions having logFC <-1 in the second set.
- Retrieve the RepeatMasker annotation for the chosen genome as a bed file and count the number of elements in each set. Convert the count as a ratio over the number of regions for each dataset.
- Extract the ratio of methylome that overlaps each class of repeats and perform a Chi-squared test to detect any significant enrichment.
- Evaluate if differentially transcribed or differentially methylated regions between ETR- and mock-treated samples map to in silico-predicted off-target gRNA binding.
- Use CRISPR design suite52 as the off-target gRNA binding prediction tool.
- For every putative off-target region, look at the closest transcription start site (TSS) and the closest methylated region. Consider as a true off-target effect a region associated either to a gene regulated with FDR <0.01 and a distance to TSS smaller than 10 Kb, or a methylated region regulated with FDR <0.01 and a distance lower than 1 Kb.
- Identify the number and features of the regions transcriptionally altered or overmethylated in samples treated with each of the three top-performing gRNAs compared to mock-treated samples (Figure 5) to identify the most specific among the gRNAs. Rank the potential impact of an off-target site on the physiology of the target cells in the following order (from the most impacting to the less impacting):
i) Intragenic, regulatory region-physiologically expressed gene
ii) Intragenic, exonic region-physiologically expressed gene
iii) Intragenic, intronic region-physiologically expressed gene
iv) Intragenic, regulatory region-not expressed gene
v) Intragenic, exonic region-not expressed gene
vi) Intragenic, intronic region-not expressed gene
vii) Intergenic region
Results
Upon delivery of a donor template for homologous recombination-mediated integration of the fluorescent reporter in the target locus coupled with the CRISPR/Cas9 system (e.g., by plasmid nucleofection in the case of K-562 cells), reporter-positive cells appear in the treated sample (Figure 1, bottom). If this does not occur, recheck the accuracy of design and cloning of both the donor template and CRISPR/Cas9 reagents. If confirmed, try to optimize the doses of reagents and the delivery protocol itself.
Once the reporter cell line is obtained, select promoter/enhancer sequences of the target gene and design a panel of matching gRNAs. Use in silico prediction tools such as Chopchop to both identify the gRNA sequences and rank them in terms of predicted efficiency and specificity (Figure 2). If no gRNAs are retrieved, control the presence of the canonical Cas9 protospacer adjacent motif (PAM) (5'-NGG-3') in the target sequence. If no PAM sequences are present, consider either shifting to ETRs based on alternative PAM-independent Cas9 variants (no ETRs published with these variants yet) or switching to alternative DNA binding domain platforms, such as ZFPs53 or TALEs30. However, if only gRNAs with low predicted efficacy/specificity are retrieved, consider either 1) testing these low-quality gRNAs or 2) expanding the target DNA sequence, in search for better gRNAs. Upon transient delivery of the triple ETR combination (or CRISPRoff; v2.1) together with gRNAs, perform longitudinal flow cytometry analyses for the expression of the fluorescent reporter, often observing a peak in reporter repression at acute analyses, which is then at least partially reabsorbed due to mitotic dilution of the ETR-encoding plasmids over time (Figure 4C). If the ETRs/gRNA combination effectively deposits CpG methylation on the target locus, permanent repression of the reporter will occur in a sizable fraction of treated cells (Figure 4C). Different gRNAs can show variable long-term silencing efficiency (Figure 4B,C).
For genome-wide specificity assessments, use MeDIP-seq to identify the differentially methylated regions between cells in which the target has been long-term silenced and untreated cells. Ideally, a highly specific gRNA will only induce a peak of de novo CpG methylation at the target site (Figure 5). If not, one can consider characterizing the off-target activity of the gRNAs ranked in a lower position in the on-target efficiency list.
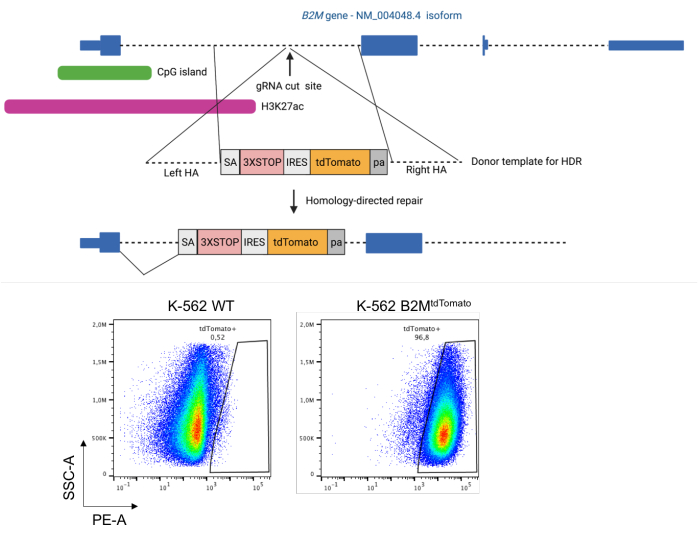
Figure 1: Integration of a tdTomato reporter under the regulatory elements of the human B2M gene by homology-directed repair. Top: Schematics of the strategy to integrate a tdTomato fluorescent reporter in the first intron of the human B2M gene by CRISPR/Cas9-induced homology-directed repair. Bottom: representative dot plots of K-562 cells pre- and post-integration of the tdTomato reporter in the first intron of the B2M gene. Abbreviations: HA = homology arm; HDR = homology-directed repair; IRES = internal ribosome entry site; pa = BGH poly(A); SA = splice acceptor site; WT = wild type; 3XSTOP = three in-tandem stop codons. Please click here to view a larger version of this figure.
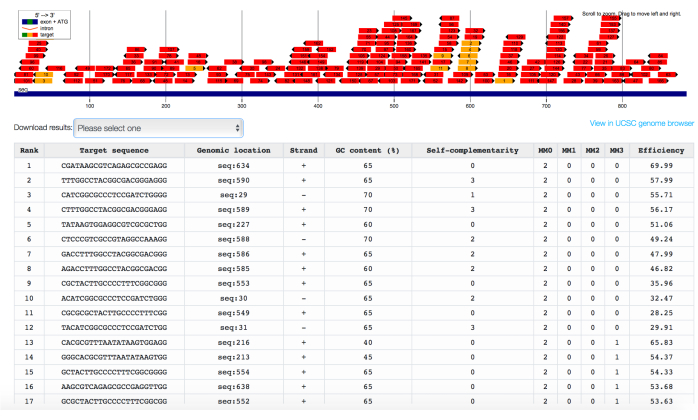
Figure 2: In silico identification of gRNAs targeting the CpG island of the B2M gene, ranked for predicted efficiency and specificity. Chopchop's output interface showing gRNAs targeting the CpG island embedded in promoter sequence of the B2M gene, ranked for both the number of off-target sequences with 0 (MM0), 1 (MM1), 2 (MM2), or 3 (MM3) mismatches and the predicted on-target efficiency. Please click here to view a larger version of this figure.
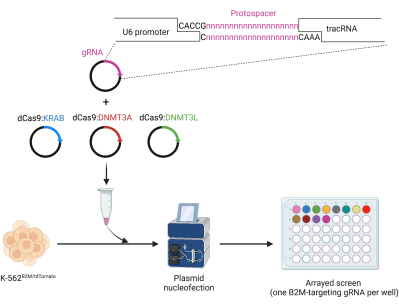
Figure 3: Cloning and arrayed nucleofection of gRNAs for dCas9 ETR-mediated epigenetic silencing. Top: oligo-mediated protospacer cloning in a human U6-gRNA expressing plasmid. Bottom: arrayed screen of B2M-targeting gRNAs for CRISPR/dCas9-based epigenetic silencing by plasmid nucleofection in K-562B2M/tdTomato cells. Please click here to view a larger version of this figure.
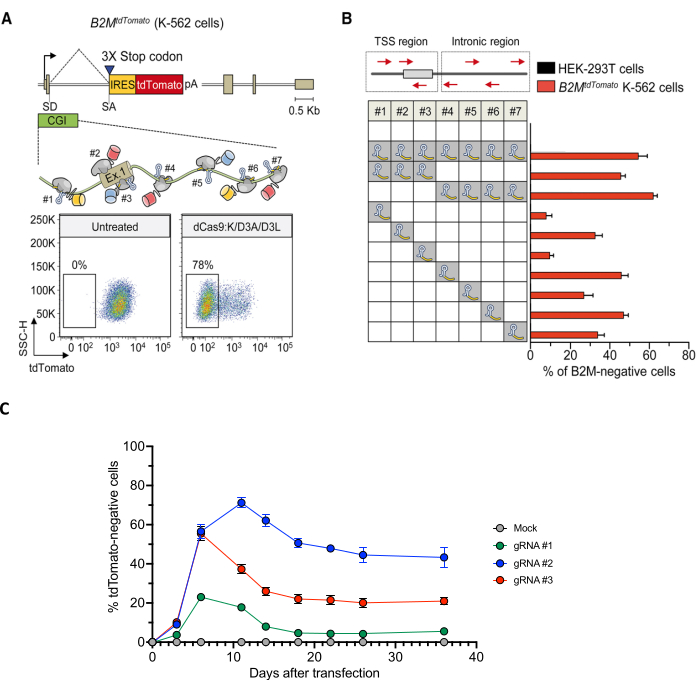
Figure 4: Screen for gRNAs effectively inducing long-term silencing of the B2M gene. (A) Top: schematics of the B2MtdTomato gene depicted in the enlarged area, the relative order and orientation of binding of dCas9-based ETRs complexed with gRNAs. Bottom: representative dot plots of B2MtdTomato K-562 cells either before (left) or after (right) ETR silencing. Analyses at 30 days post-transfection with plasmids encoding for the gRNAs and the triple dCas9:KRAB+ dCas9:D3A + dCas9:D3L combination. (B) Silencing activity of the indicated gRNAs (either in pools or as individual gRNAs) targeting the CpG island of B2M (red arrows in the top schematic indicate orientation of the gRNAs) in K-562 B2MtdTomato cells at day 30 post-transfection. Data show the percentage of tdTomato-negative cells (mean ± SEM; n = four independent transfections for each treatment condition). (C) Time-course analysis of B2MtdTomato K-562 cells upon transfection with plasmids expressing the triple ETR combination and the indicated B2M CpG island-targeting gRNAs or mock (gRNA- and ETR-free transfection). Data show the percentage of tdTomato-negative cells (mean ± SEM; n = three independent transfections for each treatment condition). Unpublished data. Panels A and B adapted from Amabile et al.30. Abbreviations: CGI = CpG island; IRES = internal ribosome entry site; pa = BGH poly(A); SA = splice acceptor site; SD = splice donor site; TSS = transcription start site; UT = untransfected; 3XSTOP = 3 in-tandem stop codons. Please click here to view a larger version of this figure.
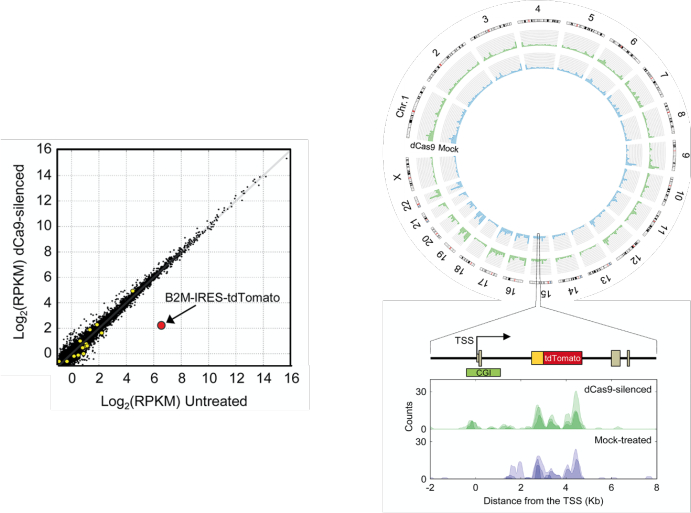
Figure 5: Genome-wide analysis of the ETR specificity by RNA-seq and by MeDIP-seq. Left: comparison of expression levels in mock-treated B2MtdTomato K-562 cells and cells treated with the triple dCas9:KRAB, dCas9:DNMT3A, dCas9:DNMT3L ETR combination and with a gRNA targeting the CpG island of the B2M gene. Values are expressed in log2 of reads per kilobase per million (RPKM) of mapped reads. Black dots represent genes expressed at comparable levels in all conditions; yellow circles represent genes differentially regulated under an FDR <0.01; red circle represents the B2M-IRES-tdTomato transcript. Top right: circos plot showing whole-genome MeDIP-seq profiles of mock-treated B2MtdTomato K-562 cells (blue) or cells treated with the triple dCas9:KRAB, dCas9:DNMT3A, dCas9:DNMT3L ETR combination and with a gRNA targeting the CpG island (CGI) of the B2M gene (green). Bottom right: the methylation status of the B2MtdTomato locus in the indicated samples is shown. Three replicates are represented in each pileup; the pileup of aligned reads were smoothed using a gaussian window. This figure was adapted from Amabile et al.30. Abbreviation: TSS = transcription start site. Please click here to view a larger version of this figure.
Discussion
Targeted epigenetic silencing may represent a promising solution to treat disorders that can benefit from permanent gene inactivation, including diseases caused by gain-of-function mutations1, infectious diseases2, and pathologies in which silencing of one gene may either compensate for an inherited defect in another one3 or unleash the full potential of adoptive cell therapies4,5. By acting at the chromatin level and being auto-propagated by the cell7,30,32, epigenetic silencing can avoid toxic alterations (e.g., chromosomal rearrangements) of the DNA sequence of the target gene and partial, transient silencing of the target, which are limitations of artificial nuclease-based gene disruption8,34,35 and RNAi-based knockdown33, respectively.
One of the key preliminary steps in any epigenetic silencing protocol is to identify the proper position on the target gene to direct the ETRs that will deposit the repressive epigenetic marks necessary to turn off the transcriptional activity of the target. Different transcriptional start site-proximal and -distal regulatory elements may concur to support the transcriptional output of a given human gene54. Further, different target sites per specific regulatory element can now be identified thanks to the growing number of programmable DNA binding technologies6. Therefore, protocols, such as the one described here in engineered cell lines, can be used to nominate individual target sites and/or genomic regions amenable to ETR-mediated epigenetic silencing, before embarking in cumbersome and time-consuming evaluation efforts of the best candidates in the final therapeutic setting. Some critical aspects of the protocol are further described below.
Engineering of a reporter cell line predictive of the final therapeutic target
Despite the constant optimization of cell engineering protocols-required here to insert the cassette coding for the fluorescent reporter in the target gene and to deliver the ETRs-it cannot be taken for granted that they might be already available for the cell line that most resembles the final therapeutic target most. In this case, different mitigation strategies can be applied: a) taking advantage of optimization kits provided by vendors to optimize in house the transfection protocol for the target cell line; b) switching to other cell lines still expressing that target gene but belonging to tissues other than the final therapeutic target-for which engineering protocols have been extensively optimized. In case these options are not available, one can consider switching to primary cell types or organoids representing the final target. As a general consideration for both preclinical studies and therapeutic applications of ETR-based epigenetic silencing, scenarios in which the target gene is essential for the target cell type should be discarded. The full, long-term silencing of an essential gene imposed by the ETRs will lead to counterselection of the target cells over time (and, potentially, toxicity of the treatment). Alternative technologies providing partial target abrogation, such as RNAi33, are preferred in these cases.
Evaluation of the on-target silencing activity of the ETRs
ETRs based on the combination of KRAB, DNMT3A, and DNMT3L effector domains have proven to be effective against the large majority of protein-coding genes, with a wide-around 1 kilobase long-permissive targeting window centered on the transcription start site32. To get a sense of how a well-performed epigenetic silencing experiment looks, the technical details and results of silencing the B2M gene in K-562 cells are provided here. This can be considered an important positive control to be included not only by researchers working with K-562 cells but also by those approaching to the ETR-based technology for the first time. As stated in the protocol, artificial nuclease (e.g., CRISPR/Cas9)-based gene disruption is recommended as an additional control of both the efficiency of gene delivery in the cell type of interest and of the phenotype of cells deprived of the target gene. After the initial screen of gRNAs to be coupled with the CRISPR/dCas9-based ETRs, if none of the gRNAs tested are able to permanently silence the target gene, one should consider, in the following order: 1) increasing the amount of gRNAs and ETRs delivered; 2) testing pools of the top gRNAs looking for synergistic effects between them. If long-term silencing is still not achieved, one should consider: 3) testing additional gRNAs, which may target sites more relevant to instruct epigenetic silencing; 4) switching to ZFP8- or TALE9-based DBD platforms, which may have an improved binding capacity to the target chromatin; 5) switching from transient to stable-for example, integrating viral vector-based-expression of the ETRs (either the gRNA or the dCas9 fusion constructs or both when adopting the CRISPR/dCas9 technology). Since our group developed, and have robust experience with, the co-delivery of the three separate ETRs30, the protocol and results shown here are based on this approach. However, a similar conceptual workflow can be likely applied for an all-in-one CRISPR-based system32.
Evaluation of the off-target activity of the ETRs
Multiple studies have shown preliminary in vitro indications of the specificity of ETRs based on the combination of KRAB, DNMT3A, and DNMT3L effector domains30,31,32. However, if among the gRNAs tested, none of them show a satisfactory specificity profile in terms of transcriptional regulation and/or de novo DNA methylation, one may pursue two non-mutually exclusive strategies: a) reducing the residence time of the ETRs inside the cell (and consequently their potential off-target activity) by either decreasing the doses of ETRs or testing alternative delivery systems. For instance, compared to plasmids, both mRNA and protein delivery are expected to reduce the cell-exposure time to the ETRs and, consequently, the likelihood of off-target activity55; b) switching to more recent Cas9 variants, optimized to reduce the off-target binding of the platform56, or to alternative ZFP8- or TALE9-based DNA binding technologies. It is important to consider that, compared to mock-treated samples, the on-target and the off-target activity of gene silencing are affected not only by the binding of the DBD to their target sequence but also by the potential capacity of the epigenetic effector domains to be recruited to other loci by their natural, endogenous cofactors. Therefore, reducing the residence time of the ETRs in the target cell may decrease not only the likelihood of binding of the DBD to off-target sites, but also the likelihood of the ETRs to interact with endogenous cofactors, with potential benefits in terms of specificity and disadvantages in terms of on-target activity. Finally, compared to mock-treated samples, some of the transcriptional and-less likely-CpG methylation alterations measured in silenced cells can simply be derived by the deprivation of the target gene. These are not considered off-targets of the silencing technology. To identify them, one should also include gene disruption by artificial nuclease in the experimental panel8,9,10. Biological alterations due to the functional loss of the target gene will be shared between epigenetic silencing and this alternative technology.
Disclosures
AL is a co-founder, quota holder, and consultant of Chroma Medicine, Inc.
Acknowledgements
The authors want to thank Angelo Amabile, Paola Capasso, Ilaria Caserta, Tania Baccega, Alice Reschigna, Valeria Mollica, and Deborah Cipria for the collaborative effort at developing the epigenetic silencing technology throughout the years; Dejan Lazarevic and Francesca Giannese for critical review of the RNA-seq and MeDIP-seq analyses described in the protocol. This work was supported by grants to A.L. from Telethon Foundation (TIGET grant no. F1) and the EU Horizon 2020 Program (UPGRADE). Illustrations have been created with BioRender.com.
AUTHORS CONTRIBUTION
A.M, M.A.C., F.G. and A.C. contributed to design of the protocol and to write the manuscript; S.V., I.M. and D.C. designed the bioinformatics sections of the protocol and revised the manuscript; A.M. and A.L. designed the protocol, conceived and wrote the manuscript with input from all the authors.
Materials
| Name | Company | Catalog Number | Comments |
| 4200 TapeStation System | Agilent | G2991BA | DNA quantification |
| 4D-Nucleofector X Unit | Lonza Bioscience | AAF-1003X | Nucleofection |
| B2M silencing gRNA #1 | Lombardo's lab | GCAATCAGGACAAGGCCCGC | Gene silencing |
| B2M silencing gRNA #2 | Lombardo's lab | GGGGTAGGAGAGACTCACGC | Gene silencing |
| B2M silencing gRNA #3 | Lombardo's lab | GAGTCCAGGGCTGGATCTCG | Gene silencing |
| BD FACSAria Fusion Flow Cytometer | BD Biosciences | https://www.bdbiosciences.com/en-us/products/instruments/flow-cytometers/research-cell-sorters/bd-facsaria-fusion | Fluorescence Activated Cell Sorting |
| bedtools | Bedtools | http://bedtools.readthedocs.io/en/latest/ | Processing of genomic intervals |
| bwa | Ih3 | https://github.com/lh3/bwa | Alignment of MeDIP-seq reads |
| Chopchop | Valen's lab | http://chopchop.cbu.uib.no/ | gRNA selection software |
| Corning RPMI 1640 Medium (Mod.) 1x with L-Glutamine | Corning | 10-040-CV | Cell culture |
| cqn | Bioconductor | http://bioconductor.org/packages/release/bioc/html/cqn.html | Region-wise normalization by GC-content |
| CRISPR design suite | Zhang's lab | https://zlab.bio/resources-2 | off-target gRNA binding prediction |
| CRISPRoff-v2.1 plasmid | Addgene | 167981 | Gene silencing |
| CytoFLEX S V4-B4-R3-I2 Flow Cytometer | Beckman Coulter | C01161 | Flow cytometry |
| Donor template sequence for tdTomato integration in the first intron of the B2M gene | Lombardo's lab | ctcctcctctgacctgtgtgtgggttttgtttttgtttt | Genetic engineering |
| E220 Focused-ultrasonicator | Covaris | 500239 | DNA sonication |
| edgeR | Bioconductor | https://bioconductor.org/packages/release/bioc/html/edgeR.html | Differential abundance testing |
| EnGen Mutation Detection Kit | NEB | E3321 | Gene disruption quantification |
| Fetal Bovine Serum | Sigma-Aldrich | F2442 | Cell culture |
| Flowjo | BD Biosciences | https://www.flowjo.com/solutions/flowjo | Flow cytometry data analysis software |
| Gel Loading Dye, Purple (6x) | NEB | B7024S | DNA gel loading |
| Go Taq G2 Hot Start DNA Polymerase | Promega | M7401 | PCR amplification |
| gRNA sequence for dTomato integration in the first intron of the B2M gene | Lombardo's lab | AGGCTACTAGCCCCATCAAG | Genetic engineering |
| hCas9 plasmid | Addgene | 41815 | Genetic engineering |
| High Sensitivity D1000 Reagents | Agilent | 5067-5585 | DNA quantification |
| High Sensitivity D1000 ScreenTape | Agilent | 5067-5584 | DNA quantification |
| High Sensitivity RNA ScreenTape | Agilent | 5067-5579 | RNA quantification |
| High Sensitivity RNA ScreenTape Ladder | Agilent | 5067-5581 | RNA quantification |
| High Sensitivity RNA ScreenTape Sample Buffer | Agilent | 5067-5580 | RNA quantification |
| IPure kit | Diagenode | C03010011 | Purification of the 5-methylcytosine immunoprecipitation product |
| K-562 cells | ATCC | CCL-243 | Cell engineering |
| KAPA Library Quantification Kit | Roche | KK4824 | MeDIP-Seq libraries preparation |
| MACS2 | Taoliu | https://github.com/macs3-project/MACS | Identification of methyl-enriched regions |
| MagMeDIP kit | Diagenode | C02010020 | 5-methylcytosine immunoprecipitation |
| NextFlex Methylseq kit 1 | Bioo Scientific | 5118-01 | MeDIP-Seq libraries preparation |
| NextSeq 500 / NovaSeq 6000 | Illumina | SY-415-1002 / 20012850 | Next Generation Sequencing |
| NucleoBond Xtra Midi kit for transfection-grade plasmid DNA | Macherey-nagel | 740410.50 | Midiprep plasmid preparation |
| One Shot TOP10 Chemically Competent cells | ThermoFisher | C404010 | Plasmid transformation |
| pAC154-dual-dCas9VP160-sgExpression plasmid | Addgene | 48240 | Gene activation |
| pcDNA.CMV.dCas9:KRAB plasmid | Lombardo's lab | available on request (lombardo.angelo@hsr.it) | Gene silencing |
| pcDNA.CMV.dCas9:KRAB plasmid | Lombardo's lab | available on request (lombardo.angelo@hsr.it) | Gene silencing |
| pcDNA.CMV.dCas9:KRAB plasmid | Lombardo's lab | available on request (lombardo.angelo@hsr.it) | Gene silencing |
| Penicillin/Streptomycin | Sigma-Aldrich | P0781 | Cell culture |
| phU6.sgRNA plasmid | Addgene | 53188 | Genetic engineering |
| PowerPac Basic Power Supply | Biorad | 1645050 | Agarose gel electrophoresis |
| Primer forward sequence to check tdTomato integration in the first intron of the B2M gene | Lombardo's lab | GTATTTGCTGGTTATGTTAG | Genetic engineering |
| Primer reverse sequence to check tdTomato integration in the first intron of the B2M gene | Lombardo's lab | AATGGTTGAGTTGGAC | Genetic engineering |
| QIAamp DNA Mini Kit | Qiagen | 51304 | DNA extraction |
| Qubit | Thermo Fisher | Q33238 | DNA quantification |
| Qubit dsDNA HS (high sensitivity) Assay Kit | Thermo Fisher | Q32851 | DNA quantification |
| Qubit RNA HS (high sensitivity) Assay Kit | Thermo Fisher | Q32852 | RNA quantification |
| Restriction enzymes | NEB | DNA digestion | |
| RNeasy Mini kit | Qiagen | 74106 | RNA extraction |
| Rsubread | Bioconductor | https://bioconductor.org/packages/release/bioc/html/Rsubread.html | Quantification of gene expression |
| SF Cell Line 4D-Nucleofector X Kit S (32 RCT) | Lonza Bioscience | V4XC-2032 | Nucleofection |
| SnapGene | Dotmatics | https://www.snapgene.com/ | Molecular biology design software |
| STAR | Alexander Dobin | https://github.com/alexdobin/STAR | Alignment of RNA-seq reads |
| T100 Thermal Cycler | Biorad | 1861096 | PCR amplification |
| T4 DNA Ligase | Promega | M1801 | DNA ligation |
| TAE Buffer | Fisher scientific | BP1332500 | Agarose gel electrophoresis |
| TruSeq Stranded Total RNA kit | Illumina | 20020597 | RNA-Seq library preparation |
| UltraPure Agarose | ThermoFisher | 16500500 | Agarose gel |
References
- Cummings, C. J., Zoghbi, H. Y. Fourteen and counting: unraveling trinucleotide repeat diseases. Human Molecular Genetics. 9 (6), 909-916 (2000).
- Tebas, P., et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England Journal of Medicine. 370 (10), 901-910 (2014).
- Frangoul, H., et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. The New England Journal of Medicine. 384 (3), 252-260 (2021).
- Murty, T., Gene Mackall, C. L. Gene editing to enhance the efficacy of cancer cell therapies. Molecular Therapy. 29 (11), 3153-3162 (2021).
- Lanza, R., Russell, D. W., Nagy, A. Engineering universal cells that evade immune detection. Nature Reviews Immunology. 19 (12), 723-733 (2019).
- Matharu, N., Ahituv, N. Modulating gene regulation to treat genetic disorders. Nature Reviews Drug Discovery. 19 (11), 757-775 (2020).
- Sgro, A., Blancafort, P. Epigenome engineering: New technologies for precision medicine. Nucleic Acids Research. 48 (22), 12453-12482 (2020).
- Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S., Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nature Reviews Genetics. 11 (9), 636-646 (2010).
- Joung, J. K., Sander, J. D. TALENs: A widely applicable technology for targeted genome editing. Nature Reviews Molecular Cell Biology. 14 (1), 49-55 (2013).
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nature Communications. 9 (1), 1911 (2018).
- Gilbert, L. A., et al. XCRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 154 (2), 442 (2013).
- Snowden, A. W., Gregory, P. D., Case, C. C., Pabo, C. O. Gene-specific targeting of H3K9 methylation is sufficient for initiating repression in vivo. Current Biology. 12 (24), 2159-2166 (2002).
- Chen, X., et al. Construction and validation of the CRISPR/dCas9-EZH2 system for targeted H3K27Me3 modification. Biochemical and Biophysical Research Communications. 511 (2), 246-252 (2019).
- Kwon, D. Y., Zhao, Y. T., Lamonica, J. M., Zhou, Z. Locus-specific histone deacetylation using a synthetic CRISPR-Cas9-based HDAC. Nature Communications. 8, 15215 (2017).
- Stepper, P., et al. Efficient targeted DNA methylation with chimeric dCas9-Dnmt3a-Dnmt3L methyltransferase. Nucleic Acids Research. 45 (4), 1703-1713 (2017).
- Ecco, G., Imbeault, M., Trono, D. KRAB zinc finger proteins. Development. 144 (15), 2719-2729 (2017).
- Witzgall, R., O'leary, E., Leaf, A., Onaldi, D., Bonventre, J. The Kruppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proceedings of the National Academy of Sciences. 91 (10), 4514-4518 (1994).
- Mannini, R., et al. Structure/function of KRAB repression domains: Structural properties of KRAB modules inferred from hydrodynamic, circular dichroism, and FTIR spectroscopic analyses. Proteins: Structure, Function and Genetics. 62 (3), 604-616 (2006).
- Friedman, J. R., et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes and Development. 10 (16), 2067-2078 (1996).
- Iyengar, S., Farnham, P. J. KAP1 protein: An enigmatic master regulator of the genome. The Journal of Biological Chemistry. 286 (30), 26267-26276 (2011).
- Schultz, D. C., Friedman, J. R., Rauscher, F. J. Targeting histone deacetylase complexes via KRAB-zinc finger proteins: The PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2α subunit of NuRD. Genes and Development. 15 (4), 428-443 (2001).
- Schultz, D. C., Ayyanathan, K., Negorev, D., Maul, G. G., Rauscher, F. J. SETDB1: A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes and Development. 16 (8), 919-932 (2002).
- Nielsen, A. L., et al. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. The EMBO Journal. 18 (22), 6385-6395 (1999).
- Sripathy, S. P., Stevens, J., Schultz, D. C. The KAP1 corepressor functions to coordinate the assembly of de novo HP1-demarcated microenvironments of heterochromatin required for KRAB zinc finger protein-mediated transcriptional repression. Molecular and Cellular Biology. 26 (22), 8623-8638 (2006).
- Jurkowska, R. Z., Jurkowski, T. P., Jeltsch, A. Structure and function of mammalian DNA methyltransferases. ChemBioChem. 12 (2), 206-222 (2011).
- Jia, D., Jurkowska, R. Z., Zhang, X., Jeltsch, A., Cheng, X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature. 449 (7159), 248-251 (2007).
- Tajima, S., Suetake, I., Takeshita, K., Nakagawa, A., Kimura, H. Domain structure of the Dnmt1, Dnmt3a, and Dnmt3b DNA methyltransferases. Advances in Experimental Medicine and Biology. 945, 63-86 (2016).
- Greenberg, M. V. C., Bourc'his, D. The diverse roles of DNA methylation in mammalian development and disease. Nature Reviews Molecular Cell Biology. 20 (10), 590-607 (2019).
- Ishiyama, S., et al. Structure of the Dnmt1 reader module complexed with a unique two-mono-ubiquitin mark on histone H3 reveals the basis for DNA methylation maintenance. Molecular Cell. 68 (2), 350.e7-360.e7 (2017).
- Amabile, A., et al. Inheritable silencing of endogenous genes by hit-and-run targeted epigenetic editing. Cell. 167 (1), 219.e14-232.e14 (2016).
- Mlambo, T., et al. Designer epigenome modifiers enable robust and sustained gene silencing in clinically relevant human cells. Nucleic Acids Research. 46 (9), 4456-4468 (2018).
- Nuñez, J. K., et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell. 184 (9), 2503.e17-2519.e17 (2021).
- Davidson, B. L., McCray, P. B. Current prospects for RNA interference-based therapies. Nature Reviews Genetics. 12 (5), 329-340 (2011).
- Haapaniemi, E., Botla, S., Persson, J., Schmierer, B., Taipale, J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nature Medicine. 24 (7), 927-930 (2018).
- Kosicki, M., Tomberg, K., Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nature Biotechnology. 36 (8), 765-771 (2018).
- Ciccia, A., Elledge, S. J. The DNA damage response: making it safe to play with knives. Molecular Cell. 40 (2), 179-204 (2010).
- Hu, J. H., et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 556 (7699), 57-63 (2018).
- Uhlén, M., et al. Proteomics. Tissue-based map of the human proteome. Science. 347 (6220), (2015).
- Labun, K., et al. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Research. 47 (W1), W171-W174 (2019).
- Kent, W. J., et al. The Human Genome Browser at UCSC. Genome Research. 12 (6), 996-1006 (2002).
- Cheng, A. W., et al. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Research. 23 (10), 1163-1171 (2013).
- Kabadi, A. M., Ousterout, D. G., Hilton, I. B., Gersbach, C. A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Research. 42 (19), e147 (2014).
- Dobin, A., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29 (1), 15-21 (2013).
- Liao, Y., Smyth, G. K., Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research. 41 (10), e108-e108 (2013).
- Conesa, A., et al. A survey of best practices for RNA-seq data analysis. GenomeBiology. 17, 13 (2016).
- Robinson, M. D., Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology. 11 (3), R25 (2010).
- Robinson, M. D., McCarthy, D. J., Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26 (1), 139-140 (2010).
- Li, H., Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 26 (5), 589-595 (2010).
- Zhang, Y., et al. Model-based analysis of ChIP-Seq (MACS). Genome Biology. 9 (9), R137 (2008).
- Quinlan, A. R. BEDTools: The Swiss-Army tool for genome feature analysis. Current Protocols in Bioinformatics. 47, (2014).
- Hansen, K. D., Irizarry, R. A., Wu, Z. Removing technical variability in RNA-seq data using conditional quantile normalization. Biostatistics. 13 (2), 204-216 (2012).
- Hsu, P. D., et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology. 31 (9), 827-832 (2013).
- Zeitler, B., et al. Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington's disease. Nature Medicine. 25 (7), 1131-1142 (2019).
- Andersson, R., Sandelin, A. Determinants of enhancer and promoter activities of regulatory elements. Nature Reviews Genetics. 21 (2), 71-87 (2020).
- Liang, X., et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. Journal of Biotechnology. 208, 44-53 (2015).
- Han, H. A., Pang, J. K. S., Soh, B. -. S. Mitigating off-target effects in CRISPR/Cas9-mediated in vivo gene editing. Journal of Molecular Medicine. 98 (5), 615-632 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved