Method Article
Symptom Assessment of Patients with Allergic Rhinitis Using an Allergen Exposure Chamber
In This Article
Summary
A protocol for conducting a challenge in an allergen exposure chamber (AEC) facility is presented. AECs have proven to be safe and effective tools for the induction of allergic symptoms or as an endpoint in the efficacy testing of allergen immunotherapy due to their ability to maintain stable particle concentrations and environmental conditions.
Abstract
Allergen exposure chambers (AECs) are clinical facilities that allow the exposure of participants to allergenic and non-allergenic airborne particles. They provide stable particle concentrations under controlled environmental conditions. This is of great importance both for diagnostic purposes and for the monitoring of treatment effects.
Here, a protocol and the technical prerequisites for performing a safe and effective allergen challenge in subjects sensitized to airborne allergens (i.e., house dust mite [HDM]) in the ALL-MED AEC are presented. With this method, triggering allergic symptoms corresponds to natural exposure. This can be used for an allergy diagnosis or as a plausible endpoint in clinical trials, particularly for allergen immunotherapy (AIT). A controlled environment (temperature, humidity, and carbon dioxide [CO2]) in the chamber must be maintained. Allergen particles must be dispersed evenly within the AEC at stable levels throughout the challenge. For this presentation, allergic rhinitis (AR) patients sensitive to HDM allergens were enrolled. AR symptoms were assessed by the following parameters: total nasal symptom score (TNSS), acoustic rhinometry (ARM), peak nasal inspiratory flow (PNIF), and nasal secretion weight. The safety of the procedure was assessed by the peak expiratory flow rate (PEFR) and the forced expiratory volume in the first second (FEV1). The allergic subjects developed symptoms within 120 min of the trial. On average, the most intense symptoms appeared after 60-90 min and, after reaching a plateau, remained stable until the end of the trial.
Introduction
Airborne allergies are becoming a growing social problem. Proper diagnosis, the assessment of the efficacy of allergen-specific immunotherapy (AIT), and understanding the pharmacotherapies are key points in addressing this issue. However, standardizing these procedures requires stable allergen concentrations, stable environmental conditions (e.g., humidity and temperature), and the capability to cause allergic signs in a repeatable manner. Allergen exposure chambers (AECs) provide stable environmental conditions, independent of external factors, and the concentration of dispersed allergen particles is well-controlled and stable during challenges in AECs1,2.
The allergen challenge test is the basis for diagnosing airborne allergies because it provides direct evidence of a specific allergen's clinical relevance to the symptoms and severity of the allergic disease. Classic allergic diagnostics include nasal, conjunctival, and bronchial provocations3,4,5. However, the allergen challenge test in an AEC appears to be the closest to natural allergen exposure6.
This study aims to present a safe and effective method of challenging participants with various airborne allergens in an AEC to trigger significant allergic symptoms corresponding to natural exposure. This method is suitable for the induction of pathological features of respiratory diseases, including allergic rhinitis and asthma, as an endpoint in the efficacy testing of AIT and might contribute to and accelerate the clinical development of pharmacological treatments2,3,7,8,9,10.
There are over a dozen AECs in the world11. However, the AECs are not comparable to each other because they are individually designed, use different types of allergens (e.g., house dust mite [HDM], birch pollen, grass pollen, cat, ragweed pollen, or Japanese cedar pollen), and have different measurement systems for the distributed particles12,13,14,15,16,17,18,19. Therefore, each AEC should be validated for individual allergens. AEC validation ensures that the proper concentration of the allergen is safe and that the symptoms are induced in patients. The ALL-MED AEC is validated for HDM allergens20.
The ALL-MED AEC is located at the Medical Research Institute in Wroclaw, Poland. The facility can comfortably accommodate 15-20 people during one trial. The facility consists of a room with an area of 12 m2, which is accessed by an airlock to prevent particles from the external environment from entering it. The equipment (seats, walls, etc.) is comprised of non-adhesive, accessible surfaces that can be washed, such as eco-leather, plastic, and metal. The chairs are movable, allowing for different setups. The viewing window and microphone communication allow for constant monitoring of the subjects (Figure 1). Particle accumulation is measured by a laser particle counter (LPC). The particles can be categorized into different ranges, including 0-20 µm, 20-50 µm, and 50-100 µm, and the results are given in particles per cubic meter (p/m3) during a specified time unit (e.g., each minute). There are two accessory rooms next to the AEC, where patients undergo tests before entering the chamber. The rescue equipment consists of a defibrillator and other resuscitation devices housed in the facility. At least two healthcare workers, including a physician, are present during each challenge.
Protocol
This article presents a protocol that adheres to the guidelines of the Bioethics Committee at the Wroclaw Medical University in Poland. All participants were legally competent and provided written informed consent to participate in the study. They were also informed that they had the option to withdraw at any time without giving a reason.
1. Cleaning the AEC
NOTE: The cleaning can be done earlier than on the day of the experiment.
- Vacuum all the surfaces, including the furniture and the floor, with a high-efficiency particulate air (HEPA) filter vacuum cleaner.
- Clean all washable surfaces with a moisture wipe, including the furniture, the walls, the windows, and the floor.
- Turn on the compressor, which circulates air through the AEC system (allergen supply duct).
- Turn on the floor and ceiling fans so that the incoming air is regularly mixed under turbulent conditions.
- Blow the allergen supply duct with clean air for 30 min by setting the "injection length" and "break between injections " of the feeder control station to their maximum values.
- Check the contamination by the allergen by monitoring the particle number on the laser particle counter (LPC)21.
- In the main menu, press Configuration | Sample. Use the following parameters: sample for 1 min, 000 cycles, 0 min delay, hold for 0 min, and units of cubic meters (m3).
NOTE: The LPC will start counting the particles immediately and then count the particles for 1 min without an interval between each measurement. The LPC will measure the samples until manually stopped and then calculate the particles per cubic meter (p/m3). - In the main menu, press Configuration | Particles. Select all options.
NOTE: The LPC will measure all the particles up to 100 µm (full range). - Read out the result in the computer program (e.g., LMS Express 7).
NOTE: The cabin is clean when the number of particles per cubic meter (p/m3) is less than 50 p/m3 and the particles are in the range between 0-100 µm for at least 10 min.
- In the main menu, press Configuration | Sample. Use the following parameters: sample for 1 min, 000 cycles, 0 min delay, hold for 0 min, and units of cubic meters (m3).
2. Operating the AEC
NOTE: The atmosphere in the cabin must be regularly monitored by an engineer, who establishes that the parameters are constant during the trial. The parameters should be stabilized before the participants enter.
- Environment
- Turn on the compressor, which circulates air throughout the AEC.
- Adjust the temperature to 21 °C ± 0.5 °C on the temperature control system (Table of Materials).
NOTE: The temperature may vary between 18°C and 27 °C, if necessary. - Turn on the floor and ceiling swirl fans.
- Turn on the humidifier on the feeder control station (Table of Materials).
- Set the air change per hour (ACH) to be between 5 and 20 by setting the knob "air supply" on the feeder control station to the position between 40%-100%. Measure the relative humidity and CO2 concentration with an air quality meter.
NOTE: Fresh external air is drawn in via HEPA filters. Control the relative humidity (typically 40% to 58%) and carbon dioxide (CO2) concentration (below 900 parts per million [ppm]). Adjust the ACH so that both the humidity and CO2 are within the normal range. Humidity and CO2 values are very susceptible to the number of participants.
- Adjust the temperature to 21 °C ± 0.5 °C on the temperature control system (Table of Materials).
- Generation and counting of particles
NOTE: Standardized and lyophilized allergen extracts are used. The particles are injected into the air supply duct and blown into the AEC through a computer-controlled feeder. The particle concentration can be adjusted between 500/m3 and 10,000/m3. A homogeneous, spatially stable distribution of particles is obtained by turbulent mixing to ensure the allergen particles are circulating instead of falling and accumulating on the floor.- Set the LPC to count the particles for 1 min (repeat step 1.6.1).
- Set the value of the monitored particles in the range of 0-20 µm. In the main menu, press Configuration | Particles. Tick "5, 10, 20 µm". The LPC will measure all the particles in the range of 0-20 µm.
NOTE: The particles can be classified into ranges, including 0-20 µm, 20-50 µm, and 50-100 µm, if needed for monitoring a different allergen. - Put the allergen in the feeder. Set the "injection length" to 100 ms (range 10-200 ms) and the "break between injections" to 1.5 min (range 0.3-3.0 min) on the feeder control station.
NOTE: For the ALL-MED AEC validation, dried, purified Dermatophagoides pteronyssinus (Dp) mite bodies (Table of Materials) were used for the HMD challenge, and 5,000 p/m3 was the optimal concentration20. - Monitor the number of particles (p/m3). Adjust both parameters on an ongoing basis by changing their values.
- After each trial is completed, download all the measured data (p/m3, CO2 concentration) from the computer to an external drive. Analyze the data (Figure 2).
3. Security measures
- Test the participants with a PCR test for severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) 36-24 h before they enter the AEC. Only permit participants with a negative PCR result to enter the AEC.
NOTE: This step is not mandatory and depends on local coronavirus disease 2019 (COVID-19) restrictions. Patients in the cabin do not wear protective masks.
4. Examination in the cabin and clinical endpoints
NOTE: For the inclusion and exclusion criteria, as well as the participant characteristics, see Supplementary Table 1. The participants were exposed to HDM allergens at a concentration of 5,000 p/m3 for a duration of 120 min, according to the validation of the ALL-MED AEC20.
- Disinfect the participants' hands before the examinations, because the device components they touch may be a source of infection transmission. This recommendation is essential, especially during a viral disease epidemic or pandemic.
- Constantly monitor the condition of the participants through the viewing window and be in voice contact via the microphone system (Table of Materials).
- Prior to the participant entering the AEC, request that they put on disposable coveralls with a hood (Table of Materials) to guard against the infiltration of non-allergen particles and the potential contamination of clothing.
- Before the participant enters the AEC, provide them with a box containing all the necessary instrument disposable tips for use during the examination: a spirometry tip and nose plug, a disposable inspiratory flow matter mask, a peak flow meter (PFM) tip, ARM tips, a remote controller for the questionnaire, one pack of handkerchiefs, and a biohazard plastic bag for nasal secretion.
- Perform clinical endpoints following the steps below. Repeat the ARM, PNIF, PERF, and FEV1 tests before the experiment and after 60 min and 120 min. Ensure that the participants complete the TNSS survey every 30 min (Figure 3). For participant comfort, perform the tests individually in a room next to the AEC.
NOTE: For efficient testing, have the participants enter the cabin at 10 min intervals. As a result, the measurements for each subject will be taken at different real times, with each patient spending a total of 120 min inside the AEC. The time shift also allows staff members to assist and interact with the participants during the testing process. In total, the AEC operates for approximately 210 min.- Nasal secretion (objective parameter)
NOTE: The participants should have identical packages of handkerchiefs and plastic bags. This is necessary in order to compare the weights.- Instruct the participants to place any used handkerchiefs in a plastic bag. After the 2 h challenge has been completed, have the participants also place any unused handkerchiefs in the same bag. If necessary, provide additional tissues and plastic bags.
- Collect all the bags after the trial is over. Determine the weight of nasal secretions by weighing the used handkerchiefs in the plastic bags. Subtract the weight of the unused handkerchiefs and plastic bags from each measurement to obtain the weight of the nasal secretions (Figure 4A).
- Nasal symptoms survey (subjective assessment)
- Display the survey questions on the TV screen.
- Ask the patient to self-assess before the challenge and every 30 min during the challenge by selecting the number on the remote that corresponds to the severity of each symptom (question). Assess nasal symptoms based on the total nasal symptom score (TNSS) survey (Table 1).
- Send the participant an email with the TNSS questionnaire. Ask them to complete the questionnaire at home at 4 h and 24 h after the challenge and send back the results.
- After the challenge is over, download the answers, and calculate the total score for each survey (Figure 4B).
- Acoustic rhinometry (ARM) (objective parameter)
NOTE: In order to calculate the differences in minimal cross-sectional area (MCA), all the measurements of one participant must be saved in a single file. Otherwise, analysis will not be possible.- Perform the test three times: before the challenge, 60 min after the challenge, and 120 min after the challenge.
- Place the appropriate tip of the rhinometer head against the nostril (blue for the left nostril). Check if it is tight. Ask the participant to hold their breath for 3 s, and then start the program.
NOTE: In case of an unclear result, repeat the test. - Repeat for the other nostril with the appropriate tip (red for the right nostril).
- After the challenge is over, calculate the MCA (Figure 4C).
- Peak nasal inspiratory flow (PNIF) (objective parameter)
NOTE: PNIF directly measures the nasal airflow during maximal inspiration and determines the degree of nose obstruction.- Perform the test three times: before the challenge, 60 min after the challenge, and 120 min after the challenge.
- Ask the participant to deflate their lungs deeply. Then, place the disposable inspiratory flow metermask connected with the flow meter onto their face, and instruct them to breathe in through their nose to the maximum (Figure 4D).
- Make sure that the inspiratory flow meter is in a horizontal position throughout the whole test. Record the average of the best of three measurements.
- Peak expiratory flow rate PEFR (safety parameter)
NOTE: PEFR is a reliable indicator of ventilation adequacy as well as airflow obstruction.- Perform the test three times: before the challenge, 60 min after the challenge, and 120 min after the challenge.
- Ask the participant to take in as deep a breath as possible, put their lips around the peak flow matter disposable tip, and exhale quickly and forcefully (Figure 4E).
- Record the average of the best of three measurements.
- After the challenge is over, provide the participant with a PFM. Ask them to perform the test at home at 4 h and 24 h after the challenge and send back the results.
- Spirometry (safety lung parameter)
NOTE: Spirometry is performed according to the European Respiratory Society (ERS)22 standards to assess the safety and monitor possible bronchial obstruction23.- Perform the test three times: before the challenge, 60 min after the challenge, and 120 min after the challenge.
- Before the measurement, set the parameters on the spirometer for each participant: gender, age, weight, and height.
- Ask the participant to sit down and put on the nose plug. Then, have the participant place their lips around the spirometer disposable tip and breathe calmly and carefully.
- Ask the participant to take a deep breath and a strong exhalation without unnecessary delay, which can only be interrupted when the spirometer gives a signal. Repeat 3x.
- After examining all the participants, download the results, and record the forced expiratory volume in the first second (FEV1) (Figure 4F).
- Nasal secretion (objective parameter)
- If the participant's well-being or safety parameters deteriorate drastically during the allergen challenge, stop the test immediately.
- Keep the participants safe and comfortable after leaving the AEC facility by providing them with rescue medications (if needed).
- Conduct safety follow-up calls with each participant 24 h after the challenge.
Results
The AEC environment was monitored throughout the operation time for the number of allergens (p/m3), the temperature, the humidity, and the CO2 concentration (Figure 2). The HDM allergen levels were found to be stable (Figure 2A). Additionally, a trial in which no allergens were distributed is shown, with particles in the range of 0-20 µm and a particle count maximum of 50 p/m3 (Figure 2A). There was an influx of particles originating from the participants entering the AEC, resulting in about 100 p/m3 for the 15 participants compared to an empty chamber. As a result, the values measured by the LPC during the trial included the target concentration with an influx of approximately 100 p/m3.
Paired data were compared with the Mann-Whitney U test. Values were considered statistically significant for all the tests with p < 0.05. Statistical calculations were performed, and graphs were generated using a graphing program.
Two groups were included in the study to show the difference between positive and negative results: eight HDM allergic individuals with allergic rhinitis (AR) symptoms and seven healthy control (HC) individuals without allergies. Supplementary Table 1 presents the inclusion and exclusion criteria, as well as the participant characteristics. The participants were exposed to HDM at a concentration of 5,000 p/m3 for a duration of 120 min, according to the validation of the ALL-MED AEC20.
All participants underwent the following tests (ARM, PNIF, PERF, spirometry) and completed TNSS questionaries, and their nasal discharges were collected. TNSS and nasal discharge weight were significantly higher in AR individuals compared with the HC group (Figure 4A,B). The TNSS reached the peak values after 60 min of exposure and then plateaued (p < 0.0001). Additionally, the nasal secretion weight was significantly higher in the AR group (p < 0.0001). Impairment of the airway patency was noticed in the acoustic rhinometry. The MCA significantly decreased after the first measurement at 60 min when comparing the AR group to the HC group. From that point on until the end of the challenge, the values remained stable (p < 0.001). This agreed with the PNIF measurements, for which a significant reduction was observed at the same concentrations (p < 0.01) (Figure 4C,D).
The FEV1 and PEFR were measured during the AEC challenge (Figure 4E,F). Additionally, the participants measured their PEFR at home at 4 h and 24 h after the challenge and returned the results by mail. The values were within the normal range and remained stable during the challenge and for up to 24 h thereafter. No statistically significant differences were found between the allergic subjects with AR and the HCs, which suggests that the HDM allergen exposure had no effects on lung function in either group.
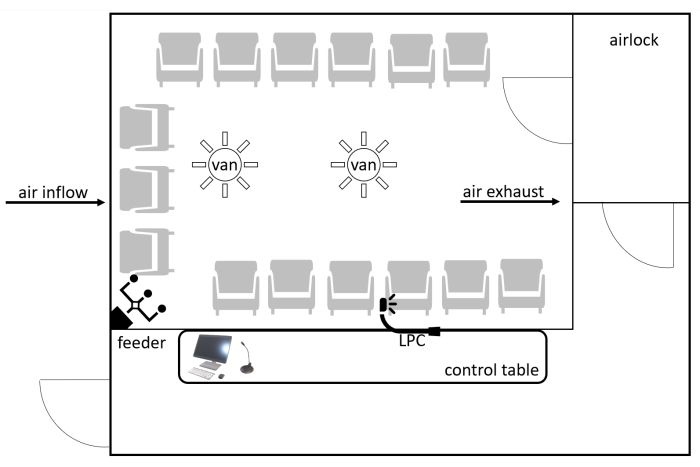
Figure 1: Schematic layout of the AEC. Participants enter through the airlock. The particles are distributed through the system of air vents by a computer-controlled feeder. The AEC conditions (particle concentration, CO2 concentration, humidity, and temperature) are constantly monitored by an LPC. The participants are monitored by the window and voice connection. Abbreviations: AEC = allergen exposure chamber; CO2 = carbon dioxide; LPC = laser particle counter. Please click here to view a larger version of this figure.
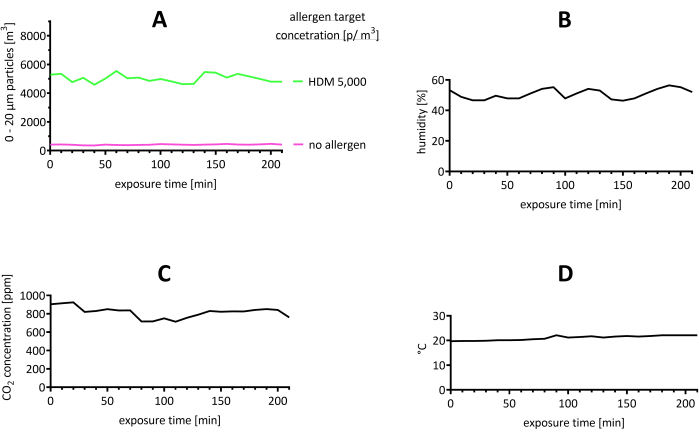
Figure 2: Representative results of the stability of the environment in the AEC during the trial. (A) The particle concentration was assessed and found to be in the range of 0-20 µm by LPC. The target value for the concentration of HDM allergens was 5,000 p/m3. For comparison, a trial where no allergen was used is shown. (B) Humidity, (C) CO2 concentration, and (D) and temperature are shown. Abbreviations: °C = degrees Celsius; CO2 = carbon dioxide; HDM = house dust mite; LPC = laser particle counter; m = meter; min = minute(s); p = particles; ppm = parts per million. Please click here to view a larger version of this figure.
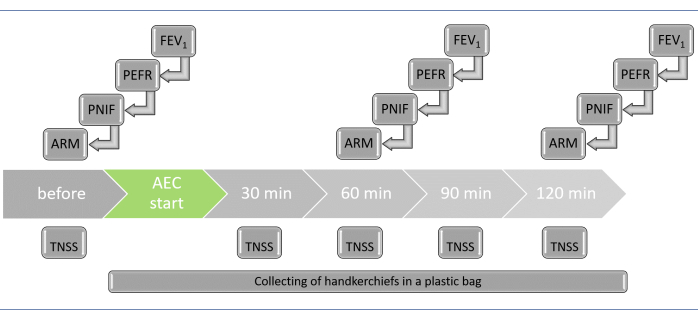
Figure 3: List of tests to be performed during the AEC challenge, with time points (for each participant). To ensure the timely execution of individual tests, the participants should enter the AEC every 10 min. As a result, the test for each participant will be conducted at different real times. Furthermore, the time shift allows the staff to help the participants during testing. Abbreviations: AEC = allergen exposure chamber; ARM = acoustic rhinometry; FEV1 = forced expiratory volume in the first second; PEFR = peak expiratory flow rate; PNIF = peak nasal inspiratory flow; TNSS = total nasal symptom score. Please click here to view a larger version of this figure.
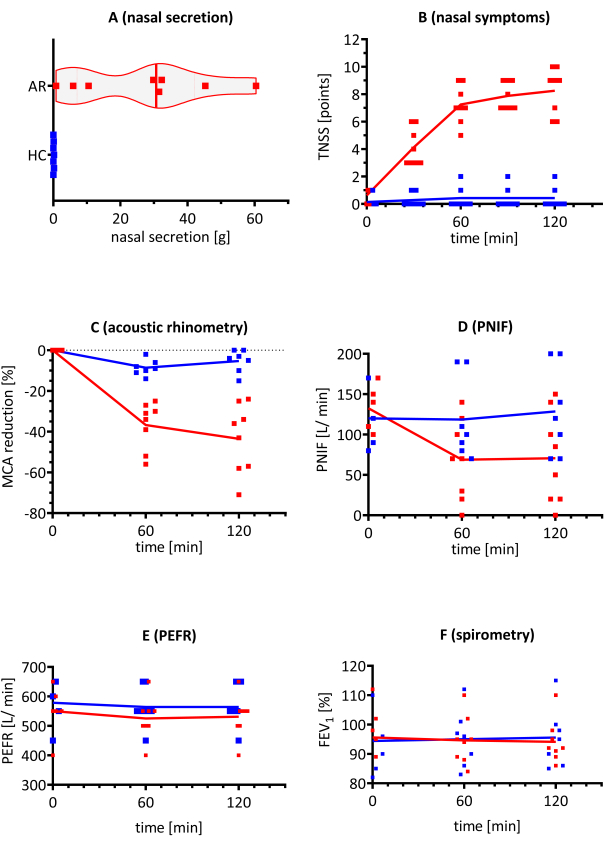
Figure 4: Representative results of different endpoints during the AEC challenge in patients with AR (red bars) and HCs (blue bars). HMD-triggered allergy subjects (with AR) and HC, including eight and seven participants, respectively, were exposed to HDM allergen concentrations of 5,000 p/m3 in the AEC. (A) Nasal secretion weight, (B) nasal symptoms, (C) MCA in acoustic rhinometry, (D) PNIF, (E) PEFR, and (F) FEV1 were evaluated. The results are presented as individual replicates with the mean value. Abbreviations: AEC = allergen exposure chamber; AR = allergic rhinitis; FEV1 = forced expiratory volume in the first second; HC = healthy controls; HDM = house dust mite; g = gram(s); MCA = minimal cross-sectional area; p = particles; PEFR = peak expiratory flow rate; PNIF = peak nasal inspiratory flow; TNSS = total nasal symptom score. Please click here to view a larger version of this figure.
| symptom | question displayed on a TV screen | TNSS score for each symptom |
| rhinorrhea | Rate how your runny nose has been at this moment | 0 = none (symptom completely absent) |
| nasal obstruction | Rate how your nasal congestion has been at this moment | 1 = mild (symptom present, but not distressing) |
| sneezing | Rate how your sneezing has been at this moment | 2 = moderate (symptom distressing, but tolerable) |
| nasal itching | Rate how your nasal itching has been at this moment | 3 = severe (symptom hard tolerable, maximum intensity) |
| 0 - 12 points total |
Table 1: Symptoms and score method for TNSS. A rating system was used by the participants to evaluate four symptoms. The survey results are presented as one value-a total score for the four questions for a given time (before the trial and every 30 min of the trial). Abbreviation: TNSS = total nasal symptom score.
Supplementary Table 1: Inclusion and exclusion criteria for the study and characteristics of the participants enrolled in the study. Eight patients with AR symptoms, triggered by HDM, and seven patients with no symptoms (HCs). Abbreviations: AR = allergic rhinitis; Df = Dermatophagoides farinae; Dp = Dermatophagoides pteronyssinus; F = female; HC = healthy control; HDM = house dust mite; kU/L = kilo units/liter; M = male; md = mean diameter; sIgE = specific immunoglobulin E; SPT = skin prick test. Please click here to download this File.
Discussion
There are a limited number of AEC facilities operating globally. A variety of allergens have been tested in these facilities, with the most common being ragweed pollen, birch pollen, grass pollen, Japanese cedar pollen, and HDM. AECs are not classified as medicinal products (according to the Directive 2001/83/EC) or medical devices (according to the Medical Device Directive 93/42/EEC)24. AECs are considered a possible tool for the measurement of primary endpoints in dose-finding studies according to the European Medicines Agency (EMA) guidelines for the development of AIT products25,26.
Critical steps in the protocol
It is essential to provide stable and sufficiently high allergen concentrations throughout the whole trial in the AEC. Research shows that AR patients do not develop allergic symptoms at low allergen concentrations20. Even moderate allergen concentrations do not trigger relevant symptoms27. Very high concentrations might cause severe reactions, such as bronchoconstriction. Therefore, optimal and sustainable allergen concentrations are key for a successful trial. Since AECs vary (as described in the introduction), each allergen used should be validated. The ALL-MED AEC is validated for the HDM allergen. It was found that the optimal endpoint for symptom assessment was 120 min, as symptoms reached a plateau after 60-90 min. The optimal challenge time and allergen concentration were selected based on challenges with different HMD concentrations at different times20. Notably, acute symptoms may occur after an allergen challenge, particularly an exacerbation of asthma.
According to the protocol, the participants complete TNSS surveys at five time points during the trial. It is essential that they do not see their previous responses in order to avoid self-suggestion. Therefore, if the questionnaires are completed on paper, the completed questionnaires should be collected immediately.
Modifications and troubleshooting of the method
Different clinical endpoints can be used depending on the symptom to be observed during the challenge (e.g., the total ocular symptom score [TOSS] to assess rhinoconjunctivitis or the non-nasal symptom score [NNSS] for respiratory system assessment).
Rhinomanometry might be used as an alternative to acoustic rhinometry. Both methods are used to test nasal patency objectively. Rhinomanometry is a standard test for the nasal cavity. It enables an objective assessment of the patency of the nasal passages by measuring the resistance in the nasal cavity during inhalation and exhalation. Acoustic rhinometry is the study of the volume of the nasal cavities. The nasal cavity's patency is assessed by an ultrasound wave. There is no data available on which method is more accurate for AEC challenges28,29.
A nasal fluid collection from a single foam sponge and specific level measurements of IgA1, IgA2, IgG, IgG, IgG4, and IgE represent additional tests that can be done during the AEC challenge30,31. Serum and peripheral blood mononuclear cells (PBMCs) can also be collected to further determine the AIT molecular mechanisms.
Patients are not allowed to use medications that may influence the onset of allergic symptoms. The most significant classes, along with the minimum times between the last dose and the AEC challenge, are antihistamines (7 days), inhaled and/or intranasal corticosteroids (14 days); inhaled and/or intranasal cromolyn (14 days), and systemic corticosteroids and/or astemizole (30 days)18.
Limitations of the method
The AEC challenge test is more expensive than direct provocation tests (nasal, conjunctival, and bronchial), which means it is not used in daily practice. AECs differ in terms of the sources of the allergen, the measurement of the distributed particles, and the trial time, which makes it very difficult to compare studies. When HDM allergens were used in the AEC, different material sources were applied: Der p 1 and Der f 1, Dp fecal material containing mainly Der p1 with a 20:1 predetermined ratio of Der p 1 to Der p 232, HDM allergen SQ 503 from body and feces containing Der p 1 and Der p 233, and Dp extracts. In the ALL-MED AEC, dried and purified Dp mite bodies, including Der p 1 and Der p 2, were used20. Therefore, unified standards should be introduced in the future so that outcomes can be compared among AECs.
The significance of the method with respect to existing/alternative methods
AECs are a very useful but underrepresented in vivo method in allergy diagnostics. Additionally, as an assessment endpoint of clinical trials, AECs show significant superiority over classical "in-field" evaluations. It is of interest to examine the correlations among various clinical endpoints, particularly the similarity of subjective parameters assessed by patients (TNSS) and objective measures (acoustic rhinometry, PNIF, nasal discharge) gathered by the investigator, as an initial step in validating AEC results against those obtained in a "field" setting.
Future applications or directions of the method
AECs offer a possible method for the stratification of patients into potential responders and non-responders. This method shows great promise for accelerating clinical developments in both the pharmacotherapy and immunotherapy of allergic diseases34. Thus, AECs have been one of the key areas of interest in recent years. AECs could be useful in long-term studies when it is not possible to evaluate the natural exposure due to low allergen counts.
Disclosures
Marek Jutel reports personal fees from ALK-Abello,Allergopharma, Stallergenes, Anergis,Allergy Therapeutics, Leti, HAL, GSK,Novartis, Teva, Takeda, and Chiesi. The other authors have nothing to disclose.
Acknowledgements
The publication was prepared under a project financed from funds granted by the Ministry of Science and Higher Education in the "Regional Initiative of Excellence" program for the years 2019-2022, project number 016/RID/2018/19, the amount of funding 11 998 121.30 PLN, and by subvention SUB.A020.21.018 of the Medical University in Wroclaw, Poland.
Materials
| Name | Company | Catalog Number | Comments |
| Allergen exposure chamber (AEC) | custom made | --- | with the air supply duct (with HEPA filters) and allergen blew into the AEC through a computer-controlled feeder |
| Acoustic rhinometer | GM Instruments (Irvine, UK) | A1 clinical/ reseach | with reusable plastic tips, contoured for the right and left nostrils |
| Air humidifier | Ohyama | SHM120D | |
| Air quality meter | AZ Instrument | Green Eye VZ 7798 | termometer, humidity and CO2 meter |
| Air-conditioning | DeLonghi | CKP 20EB | temperature range 18 - 25 °C |
| Ceiling fans | Argos | Manhattan Ceiling Fan - 432/8317 | |
| Computer-controlled feeder station | custom made | --- | with control of "injection length", "break between injections ", “air supply” |
| Disposable coveralls | VWR (Radnor, Pennsylvania, United States) | with hoodies | |
| Floor fans | AEG | TVL 5537, column | |
| Graphing program | GraphPad Software Inc. | Graph Pad Prism, v. 9.4.0 | |
| House dust mite (HDM) | Allergopharma (Reinbek, Germany) | customized order | dried, purified Dermatophagoides pteronyssinus (Dp) mite bodies, stored at 4 °C until use |
| Inspiratory flow meter | Clement Clarke International Ltd. (Harlow, UK) | portable inspiratory flow meter | with the disposable mask (size M), measuring inspiratory flow between 30 - 370 L/ min |
| Laser particle counter (LPC) | Lighthouse Worldwide Solutions (USA) | SOLAIR Boulder Counte | |
| Microphone system | Auna | VHF wireless microphone system | |
| Peak flow matter (PFM) | CareFusion (Basingstoke, UK) | MicroPeak with a standard range of 60 – 900 L/ min | with the disposable paper tips |
| Remote controls for filling questionnaires | Turning Technologies | Pilot TT ResponseCard LT, SAP: G040602A010 | a set of 32 remote controls for TT LT tests |
| Spirometer | Medizintechnik AG (Zurich, Switzerland) | EasyOne 2001, NDD | with the disposable paper tips; the spirometer should meet the ISO 26 782: 2009 standard; daily calibration of the spirometer is required |
| TV screen | Level | Level one 32" | |
| Vacuum | Siemens | extreme silencePower VSQ5X1230 | with the HEPA filters |
References
- Clark, D., Karpecki, P., Salapatek, A. M., Sheppard, J. D., Brady, T. C. Reproxalap improves signs and symptoms of allergic conjunctivitis in an allergen chamber: A real-world model of allergen exposure. Clinical Ophthalmology. 16, 15-23 (2022).
- Hossenbaccus, L., Steacy, L. M., Walker, T., Ellis, A. K. Utility of environmental exposure unit challenge protocols for the study of allergic rhinitis therapies. Current Allergy and Asthma Reports. 20 (8), 34(2020).
- Hossenbaccus, L., Ellis, A. K. The use of nasal allergen vs allergen exposure chambers to evaluate allergen immunotherapy. Expert Review of Clinical Immunology. 17 (5), 461-470 (2021).
- Schröder, J., Mösges, R. Conjunctival provocation tests: Prediction of seasonal allergy. Current Opinion in Allergy and Clinical Immunology. 18 (5), 393-397 (2018).
- Gauvreau, G. M., et al. Allergen provocation tests in respiratory research: Building on 50 of experience. European Respiratory Journal. 60 (2), 2102782(2022).
- Hohlfeld, J. M., et al. Diagnostic value of outcome measures following allergen exposure in an environmental challenge chamber compared with natural conditions. Clinical and Experimental Allergy. 40 (7), 998-1006 (2010).
- Rösner-Friese, K., Kaul, S., Vieths, S., Pfaar, O. Environmental exposure chambers in allergen immunotherapy trials: Current status and clinical validation needs. The Journal of Allergy and Clinical Immunology. 135 (3), 636-643 (2015).
- Jacobs, R. L., et al. Responses to ragweed pollen in a pollen challenge chamber versus seasonal exposure identify allergic rhinoconjunctivitis endotypes. The Journal of Allergy and Clinical Immunology. 130 (1), 122-127 (2012).
- Khayath, N., et al. Validation of Strasbourg environmental exposure chamber (EEC) ALYATEC® in mite allergic subjects with asthma. Journal of Asthma. 57 (2), 140-148 (2020).
- Bousquet, J., et al. Onset of action of the fixed combination intranasal azelastine-fluticasone propionate in an allergen exposure chamber. The Journal of Allergy and Clinical Immunology: In Practice. 6 (5), 1726-1732 (2018).
- Pfaar, O., et al. Technical standards in allergen exposure chambers worldwide - An EAACI Task Force Report. Allergy. 76 (12), 3589-3612 (2021).
- Rønborg, S. M., Mosbech, H., Poulsen, L. K. Exposure chamber for allergen challenge. A placebo-controlled, double-blind trial in house-dust-mite asthma. Allergy. 52 (8), 821-828 (1997).
- Yang, W. H., et al. Cat allergen exposure in a naturalistic exposure chamber: A prospective observational study in cat-allergic subjects. Clinical and Experimental Allergy. 52 (2), 265-275 (2022).
- Hamasaki, S., et al. Characteristics of the Chiba environmental challenge chamber. Allergology International. 63 (1), 41-50 (2014).
- Okuma, Y., et al. Persistent nasal symptoms and mediator release after continuous pollen exposure in an environmental challenge chamber. Annals of Allergy, Asthma & Immunology. 117 (2), 150-157 (2016).
- Zuberbier, T., et al. Global Allergy and Asthma European Network (GA(2)LEN) European Union Network of Excellence in Allergy and Asthma. Validation of the Global Allergy and Asthma European Network (GA2LEN) chamber for trials in allergy: Innovation of a mobile allergen exposure chamber. Journal of Allergy and Clinical Immunology. 139 (2), 1158-1166 (2017).
- Bergmann, K. C., et al. First evaluation of a symbiotic food supplement in an allergen exposure chamber in birch pollen allergic patients. World Allergy Organization Journal. 14 (1), 100494(2020).
- Ellis, A. K., Steacy, L. M., Hobsbawn, B., Conway, C. E., Walker, T. J. Clinical validation of controlled grass pollen challenge in the Environmental Exposure Unit (EEU). Allergy, Asthma, and Clinical Immunology. 11 (1), 5(2015).
- Day, J. H., Briscoe, M., Widlitz, M. D. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: Effects after controlled ragweed pollen challenge in an environmental exposure unit. Journal of Allergy and Clinical Immunology. 101 (5), 638-645 (1998).
- Zemelka-Wiacek, M., Kosowska, A., Winiarska, E., Sobanska, E., Jutel, M. Validated allergen exposure chamber is plausible tool for the assessment of house dust mite-triggered allergic rhinitis. Allergy. 78 (1), 168(2022).
- Lighthouse World Solutions. , Available from: https://www.golighthouse.com (2022).
- Graham, B. L., et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. American Journal of Respiratory and Critical Care Medicine. 200 (8), 70-88 (2019).
- Buslau, A., et al. Can we predict allergen-induced asthma in patients with allergic rhinitis. Clinical and Experimental Allergy. 44 (12), 1494-1502 (2014).
- European Medicines Agency. Guideline for clinical development of allergen immunotherapy products CHMP/EWP/18504/2006. European Medicines Agency. , (2006).
- Pfaar, O., et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: An EAACI Position Paper. Allergy. 69 (7), 854-867 (2014).
- Committee for Medicinal Products for Human Use (CHMP). Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. European Medicines Agency. , (2008).
- Krug, N., et al. Validation of an environmental exposure unit for controlled human inhalation studies with grass pollen in patients with seasonal allergic rhinitis. Clinical and Experimental Allergy. 33 (12), 1667-1674 (2003).
- Passali, D., Bellussi, L. Monitoring methods for local nasal immunotherapy. Allergy. 52 (33), 22-25 (1997).
- Keck, T., Wiesmiller, K., Lindemann, J., Rozsasi, A. Acoustic rhinometry in nasal provocation test in perennial allergic rhinitis. European Archives of Oto-rhino-laryngology. 263 (10), 910-916 (2006).
- Shamji, M. H., et al. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. Journal of Allergy and Clinical Immunology. 148 (4), 1061-1071 (2021).
- Thwaites, R. S., et al. Absorption of nasal and bronchial fluids: Precision sampling of the human respiratory mucosa and laboratory processing of samples. Journal of Visualized Experiments. (131), e56413(2018).
- Zieglmayer, P., et al. Clinical validation of a house dust mite environmental challenge chamber model. Journal of Allergy and Clinical Immunology. 140 (1), 266-268 (2017).
- Lueer, K., et al. efficacy and repeatability of a novel house dust mite allergen challenge technique in the Fraunhofer allergen challenge chamber. Allergy. 71 (12), 1693-1700 (2016).
- Pfaar, O., et al. Allergen exposure chambers: Harmonizing current concepts and projecting the needs for the future - An EAACI Position Paper. Allergy. 72 (7), 1035-1042 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved