Method Article
Mechanical Control of Relaxation Using Intact Cardiac Trabeculae
In This Article
Summary
Rapid myocardial and cardiac relaxation is essential for normal physiology. Mechanical relaxation mechanisms are now known to be dependent on strain rate. This protocol provides an overview of the acquisition and analysis of experiments to further study the mechanical control of relaxation.
Abstract
Diastolic dysfunction is a common phenotype across cardiovascular disease presentations. In addition to elevated cardiac stiffness (elevated left ventricular end-diastolic pressure), impaired cardiac relaxation is a key diagnostic indicator of diastolic dysfunction. While relaxation requires the removal of cytosolic calcium and deactivation of sarcomeric thin filaments, targeting such mechanisms has yet to provide effective treatments. Mechanical mechanisms, such as blood pressure (i.e., afterload), have been theorized to modify relaxation. Recently, we showed that modifying the strain rate of a stretch, not afterload, was both necessary and sufficient to modify the subsequent relaxation rate of myocardial tissue. The strain rate dependence of relaxation, called the mechanical control of relaxation (MCR), can be assessed using intact cardiac trabeculae. This protocol describes the preparation of a small animal model, experimental system and chamber, isolation of the heart and subsequent isolation of a trabecula, preparation of the experimental chamber, and experimental and analysis protocols. Evidence for lengthening strains in the intact heart suggests that MCR might provide new arenas for better characterization of pharmacological treatments, along with a method to assess myofilament kinetics in intact muscles. Therefore, studying the MCR may elucidate a path to novel approaches and new frontiers in the treatment of heart failure.
Introduction
Cardiac relaxation is impaired in nearly all forms of heart failure (including heart failure with reduced ejection fraction) and in many cardiovascular diseases. In addition to numerous methods to evaluate cardiac function in permeabilized muscles, the evaluation of intact cardiac muscles is gaining interest. Such tissues are assessed unloaded (ends free to contract) or loaded (length or force controlled). Historically, intact isolated myocytes have been evaluated in an unloaded condition, where the cell body is free to shorten during contraction. Intact cardiac trabeculae are often evaluated in isometric conditions, where the length is not allowed to change, but stress (force per cross-sectional area) is generated. Both intact myocyte and trabeculae methods are beginning to converge with modifications of load1,2.
Protocols for load-clamping a muscle (i.e., controlling a muscle's developed stress at a specified value that simulates physiologic afterloads) have been developed over several decades3,4,5. In intact cardiac tissues, load-clamps allow researchers to more closely mimic the in vivo cardiac cycle using isotonic or Windkessel-like afterloads6,7,8,9. The goal of this protocol is to obtain data used to quantify the MCR (i.e., the strain rate dependence of the relaxation rate)8,9.
While the MCR protocol has been adapted from prior work, the focus of this protocol (compared to similar protocols utilizing intact cardiac tissues) is on biomechanical mechanisms that modify relaxation. There are several protocols utilizing load-clamping3,4,5,7,10 and protocols focusing on Windkessel models1,2,11, but this protocol describes specifically how stretch prior to relaxation modifies the relaxation rate. We have shown that this control occurs during a proto-diastolic period8, a phase originally described by Wiggers12. In normal healthy hearts, the myocardium undergoes a lengthening strain during ejection prior to aortic valve closure (i.e., prior to isovolumic relaxation)13. This is mimicked by prolonging the duration of afterload control until the muscle begins to stretch. Clinical evidence suggests that this lengthening may be attenuated or lost in disease states14, and the implications and mechanisms of altered end-systolic strain rates have not been fully elucidated. Given the sparse treatment options for diastolic diseases and heart failure with a preserved ejection fraction, we posit that MCR may provide insight into novel mechanisms underlying impaired relaxation.
While the gross dissection described here focuses on rodents, trabecula isolation can be performed from any intact heart, and has previously been used with a human cardiac trabecula8. Similarly, the data acquisition and analysis can also be applied to cardiomyocytes or other isolated muscle types1,10. The discussion includes commentary on possible alterations and adaptations to the method, along with limitations, such as caution against utilizing papillary muscles because of the mechanical properties of the chordae9.
Protocol
The following protocol has been approved by the Institutional Animal Care and Use Committee of Wayne State University. The protocol here describes steps for utilizing rodent experimental subjects, but can be adapted for use in other model organisms.
1. Preparation
- Obtain the experimental subject and allow it to acclimate to the lab.
- Prepare 250 mL of perfusion solution and 250 mL of modified Tyrode's solution (Table 1), by oxygenating each to 10-20 ppm of O2, with pH 7.3.
- Prepare a 5 mL syringe with a cannula attached. Fill the syringe with perfusion solution. Use cannulas that are 23 G for a mouse, and 18 or 16 G for a small or large rat, respectively. Slide a 1 mm length of polyethylene (PE) tubing on the end of a blunted needle (Table of Materials), to help prevent the aorta from slipping off the cannula after being tied.
- Complete preparation of the dissection and cannulation area (Figure 1)
- Fill two clean weigh boats at least halfway with perfusion solution. Submerge the tip of the cannula in perfusion solution with at least one double-looped suture placed around the cannula. Hold the syringe in place with a bar clamp.
- Place surgical scissors, a hemostat, iris forceps, a pair of small curved scissors, and two #3 forceps for easy access.
- Prepare the experimental system (Figure 2) by priming and circulating the modified Tyrode's solution throughout the experimental system. Ensure that the chamber is completely full and stable.
- Turn on all data acquisition boxes, including the force transducer, the length motor, the pacing system, the temperature control system, and the data acquisition computer.
- Copy and rename a template folder containing the required *.dap and pointer files to reference the current experiment. Open the data acquisition software.
- Prepare the trabecula isolation tools (Vannas scissors, #5 or #55 forceps, glass probe) by submerging their tips in 10% (w/v) bovine serum albumin (BSA) in ultrapure water or perfusion solution, to coat the metal with protein (Figure 2B).
NOTE: The entire experimental system must be prepared prior to dissection.
2. Gross dissection and cannulation
- Record the identification, body weight, and other relevant information of the animal subject.
- Optionally, inject 1,000 U/kg of heparin in sterile saline to the rodent via intraperitoneal injection at least 10 min prior to euthanasia, to minimize risks of clotting before coronary perfusion.
- Place the animal in an induction chamber and induce general anesthesia using 3%-5% isoflurane vaporized in 100% oxygen, according to standard procedure.
NOTE: Turn on any external scavengers, down draft tables, or fume hoods used to minimize exposure to isoflurane. - Once the rodent loses its righting reflex and the breathing rate has slowed:
- (For a rat) remove the animal from the induction chamber and place it supine on a dissection pad, with continued anesthesia through a nose cone.
- (For a mouse) perform cervical dislocation immediately after removing the animal from the induction chamber. The nose cone is not necessary. Turn the isoflurane vaporizer to 0%.
- If needed, affix the upper limbs of the rodent to the dissection pad away from the chest wall using tape supported by pins, taking care not to pierce into the animal. Check for proper anesthesia depth (lack of toe-pinch response) prior to proceeding to the dissection.
- Using surgical scissors (Mayo for rats, Metzenbaum for mice), just below the xyphoid process, cut the skin transverse across the full width of the rodent's abdomen into the peritoneal space.
- Using surgical scissors, make two vertical cuts parasagittal (up the sides of the chest wall) on both the left and right sides of the chest wall from the transverse cut. Then, cut across the diaphragm, connecting the parasagittal cuts, and freeing the thoracic space.
- Clamp and lift the xyphoid process using a hemostat toward the rodent's head, to move the sternum and chest wall up toward the rodent's head, exposing the thoracic space and the heart. If needed, quickly extend the parasagittal cuts to near the second vertebral space, to fully expose the heart and/or break the pericardial membrane.
- Using curved iris forceps, carefully lift the heart to visualize the great vessels. Place the forceps between the myocardium and the spine of the rodent, clamp down on the greater vessels, and lift the heart, taking care not to clamp the atria or ventricular segments.
- While slightly lifting the heart, quickly place the curved iris scissors (concave up) between the curved iris forceps and the spine of the rodent, and cut the great vessels and lungs away from the heart. Quickly move the heart into a weigh boat or beaker containing fresh perfusion solution, and shake to help clear blood off of the heart.
- Turn the isoflurane vaporizer to 0%, if not already done. If large segments of lung, pericardial, or other tissues remain attached to the heart, carefully cut away and discard them at this time to minimize interference with the cannulation.
- Using the curved iris forceps, move the heart into a clean weigh boat or beaker, with the prepared cannula submerged. Cannulate the aorta, secure the aorta by tightening the looped silk suture, and flush with up to 5 mL of perfusion solution.
- Remove the heart from the cannula and place it in a silicone elastomer-coated weigh dish to prepare for trabecula isolation.
3. Isolation and equilibration of trabecula
- Place the heart in the silicone elastomer-coated dish under a stereomicroscope and illuminate it.
- Locate the right ventricular outflow tract. Pin the left atrium and the ventricular apex to the silicone elastomer in the dish.
- Using long Vannas scissors, cut from the right ventricular outflow tract to the apex along the septum. Cut from the right ventricular outflow tract to the right atrium near the aorta, then cut through the right atrium (Figure 3B).
- Using forceps, carefully pull open the right ventricular (RV) free wall from the outflow tract, being careful not to stretch the tissue.
NOTE: Experimenters may find thin, white connective tissue strands, which can be cut without concern. Larger, pink (tissue) colored strands should be evaluated with care, as they may be trabeculae that can be isolated. - Pin the free wall of the right ventricle triangle to the dish, to expose the right ventricle (Figure 3C).
- Using a glass pipette that has been melted and formed with a thin (<500 µm diameter) but not sharp end, search the exposed endocardium for free-standing trabeculae (Figure 3C).
NOTE: A free-standing trabecula is a strip of muscle that one can fully probe underneath. Use trabeculae that have parallel sides (constant width), avoiding triangular papillary muscles. Be careful during this process and subsequent dissection, as applying strain on the trabecula can cause damage, reducing the developed force. - Dissect the trabecula using small Vannas scissors. Leave a ≥1 mm cubed piece of tissue at each end of the trabecula to allow for attachment. Do not stretch the trabecula where possible, and minimize contact of metal tools, as both can also cause damage to the muscle. Relocate the pins holding the heart as needed to minimize strain on the muscle near the trabeculae identified.
- Cut ~2 inches off the end of a 7 mL transfer pipette, slowly draw the trabecula into the pipette, and transfer it into a new weigh dish containing 50% perfusion solution and 50% modified Tyrode's solution. Allow the muscle to equilibrate to the increase in extracellular calcium within the mixed solution for several minutes.
- Repeat steps 3.7-3.8 to dissect additional trabecula at this time, to obtain additional trabecula as backup.
- Turn off the pump supplying superfusion and/or suction to the experimental chamber. Using the large bore transfer pipette, move the trabecula into the experimental chamber filled with Tyrode's solution.
- Pin one >1 mm cube piece of tissue at the end of the trabecula to a hook on the force transducer, then pin the second cube to the motor.
NOTE: Separate the motor and force transducer when mounting the first side for better access to the transducer, then move the hooks as close together as possible when mounting the second cube of tissue while the trabecula remains slack. - Restart superfusion and begin pacing the muscle to determine the threshold voltage. Pace at 20% above the threshold voltage for approximately 1 h to allow the trabecula to equilibrate.
- At the end of this equilibrium period, slowly stretch the muscle using the micrometer connected to the motor until optimal developed stress generation is achieved, by observing the developed (minimum to peak) tension. Stop increasing the muscle length when the passive diastolic tension rises faster than the peak tension, indicating the optimal length has been passed.
- Turn off the transmitted microscope illumination, and illuminate the trabecula using a gooseneck illuminator at a steep angle. Using a camera connected through the microscope optics that has previously been calibrated, capture an image of the trabecula during diastole into the experimental folder. If the trabecula is wider than the camera's field of view, take multiple images across the muscle.
- Open the image(s) in an imaging software that reports pixel distances.
- Measure the pixels distance of the muscle diameter four times along its length. Measure the muscle (trabecula) length in pixels, excluding the large cube of tissue.
- If the muscle length is longer than the field of view, use reference points along the muscle to measure the entire length.
- Using the template in the experimental folder, average the diameter measures, and convert the diameter and lengths from pixels to mm using a previously obtained calibration. Calculate the cross-sectional area as π*diameter2/4 in mm2, and the muscle length in µm.
4. Data acquisition
- Once the muscle is equilibrated, open the data acquisition software, select Experiments | Length Control, and enter the calibrated trabecula length (FL) and cross-sectional area (area [m2]) in the Calibration box.
- From the template folder (Step 1.7), ensure that the freeform_file.txt points to the correct folder, and open the freeform.dap file in a text editor. Set the isotonic level (isoton) to 32,000 in the *.dap file.
- In the Length Control box, select the Freeform tab and browse to the appropriate Freeform list file. Ensure the Data Filing path is also the correct folder to save data. Begin acquiring data from fully isometric twitches by pressing Run Experiment, before acquiring load-clamp data using a feedback control with proportional and integration gain parameters.
- Acquire load-clamped data by defining the afterload (isoton) in the *.dap file, and iterate values of proportional gain (propgain) and integration (Ki) parameters by saving the file in the text editor. Press Run Experiment in the data acquisition software interface.
- Ensure that the clamp includes relengthening back to its original length (sometimes referred to as relaxation loading5) during this iteration to attain the maximal range of strain rates, by setting mode (flswitch) from one and increasing the threshold for ending the load-clamp (flthreshold) from zero.
- Control the end of the load-clamp by changing the mode (flswitch) from one to zero. Repeat the acquisition while changing the end of the load-clamp from zero relengthening to complete relengthening back to the starting length.
- To increase the length, incrementally increase the threshold for ending the load-clamp (flthreshold) from zero until the muscle nearly relengthens back to its original length.
- Reset the mode (flswitch = 1) and threshold (flthreshold = 0), and run one final data acquisition.
- If desired, modify the afterload in the study by repeating steps 4.4-4.6. This acquisition may be done immediately.
- If desired, modify the preload by stretching or shortening the muscle, or treat the muscle by adding compounds to the Tyrode's solution. If altering the preload or adding compounds, wait a minimum of 20 min to ensure that the slow-force response9,15 has stabilized and/or for the compound to fully penetrate the muscle.
- Once the data acquisition is completed, remove the trabecula. If needed, freeze or fix the trabecula for biochemical or histological analysis.
- Clean the work areas and experimental system, flush all tubing with water, and turn off all components.
5. Data analysis
- Open the data files using a quantitative analysis program by directing the program to the appropriate file path.
- Quantify relaxation by ensuring that the data analysis program analyzes the clamped beat, and that the program correctly acquires the start of the load-clamp.
- Ensure that the end of the load-clamp is identified, so that the relaxation rate (1/τ) is quantified from the peak negative derivative of stress during an isometric relaxation.
- Use the Glantz method16 to determine this exponential time constant, or other appropriate quantification of relaxation (minimum derivative of stress, logistic time constant17, or kinematic model18).
- Ensure that the data analysis program calculates the strain rate by taking the time derivative of the strain, where strain is calculated as the length as a function of time divided by length at optimal contraction.
- Repeat the above steps for all traces of a given condition.
- Plot the relationship between the relaxation rate and strain rate, limiting the maximum data to a physiological strain rate of less than 1 s-1. Exclude data at low strain rates (Figure 4C), as the relaxation phase may not reflect the exponential decay17,18.
- Obtain the slope of the line between the relaxation rate and the strain rate, and record the slope as the index of MCR.
- Repeat the above analysis for each condition interrogated.
Results
A representative data set is shown in Figure 4, and additional results can be found in prior publications8,9. Briefly, the strain rate is calculated from the derivative of the strain, just prior to isometric relaxation. Strain is the length as a function of time divided by the length of the muscle at optimal length. Relaxation rate is calculated as 1/τ, where τ is the exponential time constant16. Multiple strain rates and their resultant relaxation rates are required to determine the mechanical control of relaxation (MCR). These data are plotted on a relaxation rate versus strain rate graph. The slope of the line provides the MCR index.
Note that end systolic and diastolic strain rates are not likely to exceed 1 s-1. Therefore, the slope should only include strain rates < 1 s-1. The relaxation rate at low strain rates can be confounded by changes in the minimum time derivative of stress (dStress/dtmin), and in these cases, data from stretches less than approximately 0.15 s-1 may be ignored.
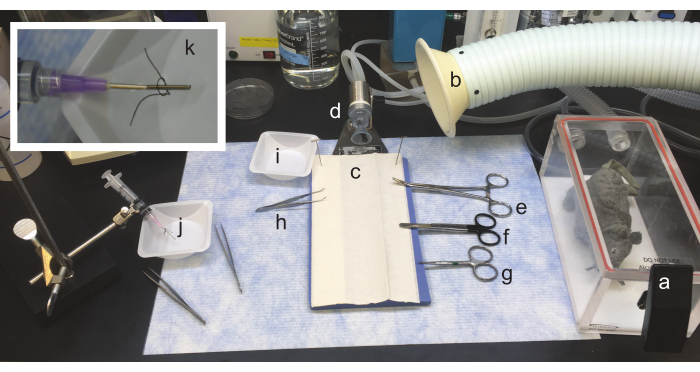
Figure 1: Setup of gross dissection and heart cannulation area. From right to left: a. The anesthesia induction chamber for the animal is located near the dissection area. b. A snorkel for optional volatile gas scavenger. c. A dissection pad, where the animal will be placed supine, is flanked by (clockwise) d. a nosecone, to provide continued anesthesia to the rodent, e. hemostats, f. surgical scissors, g. curved fine scissors placed for easy access with the dominant (cutting) hand, h. curved iris forceps placed for easy access with the non-dominant hand. Upper limbs of the rodent may be affixed to the dissection pad using tape, and i. a dissection dish (or small beaker) with perfusion solution should be placed nearby to rinse the heart. j. An area staged for cannulation must be placed nearby. A syringe with an appropriate cannula is mounted on a ring stand. k. (Inset) A closer image of a 16 G cannula, with 1 mm of PE205 tubing attached and a suture loosely knotted. Please click here to view a larger version of this figure.
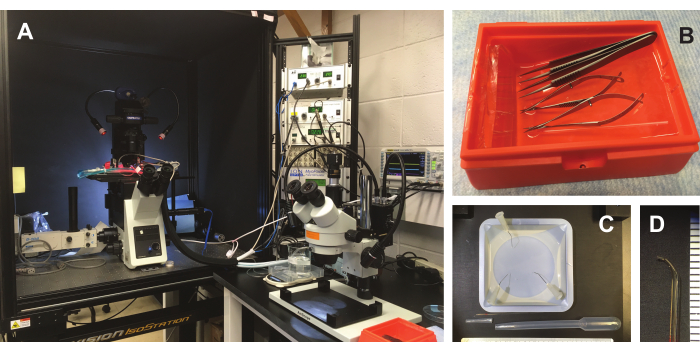
Figure 2: Experimental area and tools. (A) Experimental area. The experimental chamber and data acquisition system are prepared on a nearby inverted microscope (left). Trabecula isolation and mounting occurs under a stereoscope (right). (B) Tips of two forceps, large and small Vannas scissors, and a custom glass probe are prepared by soaking in 10% BSA solution. (C) Additional dissection tools. A silicone elastomer-plated dish allows for mounting the heart during fine dissection. A 7 mL transfer pipette with the end cut is shown below the dish and glass probe. The cut tip of the transfer pipette is discarded, and an enlarged bore is used to transfer the muscle, with minimal risk of stretching or dehydration. (D) Magnified image of the end of a glass pipette, which is approximately 2 mm long and 0.25-0.5 mm in diameter. Please click here to view a larger version of this figure.
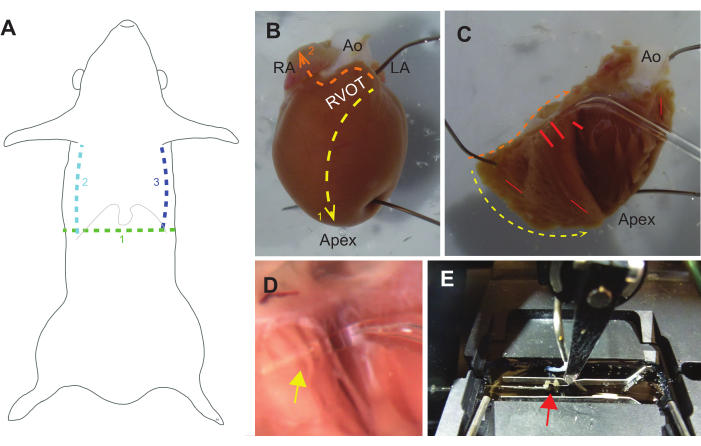
Figure 3: Guide to dissection. (A) Gross dissection should begin with a transverse cut (1. green) through the skin into the peritoneal cavity below the xyphoid process (lower ribs and xyphoid are indicated by a thin gray line). Parasagittal cuts from the peritoneal cavity up the ribcage should follow (2. teal and 3. blue lines), after which the diaphragm should be cut. A hemostat can then be used to clamp the xyphoid process and elevate the chest wall toward the head. (B) An intact rat heart pinned to a silicon-elastomer dish, oriented with a view of the right ventricular outflow tract (RVOT), right atrium (RA), aorta (Ao), and left atrium (LA). The first set of cuts should be from the RVOT to the apex along the septum (1. Yellow line). A second set of cuts should be from the RVOT along the base of the heart then across the right atrium (2. orange line). (C) The RVOT can be carefully pulled away from the aorta to open the heart and pin it back. Yellow and orange lines correspond to cuts described in B. Free standing trabeculae are often found near the base of the RV free wall and near the septum, but can occur anywhere (red lines indicate common locations). (D) Magnified view of the glass probe under a trabecula (yellow arrow indicates intersection of trabecula and probe). (E) A trabecula (red arrow) is mounted to the experimental system between a force transducer (left) and motor (center-right), and is flanked by two pacing leads (horizontal above and below the trabecula). Please click here to view a larger version of this figure.
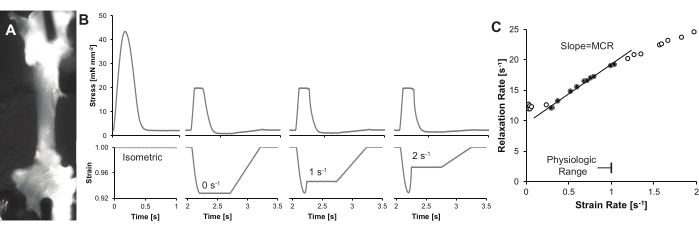
Figure 4: Representative traces and results. (A) A single cardiac trabecula illuminated using a gooseneck LED light at a steep (~75°) angle from the axis of the microscope lens, which enhances contrast of the trabecula. In this example, two images are tiled to show the full length of the trabecula. (B) Stress-time (top) and strain-time (bottom) curves for the same trabecula. An isometric twitch, along with three load-clamp twitches at increasing end systolic strain rates are shown. (C) Representative MCR calculation. MCR is defined as the slope of the line between the relaxation rate and strain rate. As noted in the discussion, strain rates may be restricted to provide a quantitative, reproducible MCR. Please click here to view a larger version of this figure.
Table 1: Solutions. Please click here to download this Table.
Discussion
Mechanical control of relaxation (MCR) quantifies the dependence of the myocardial relaxation rate on the strain rate of the muscle proceeding relaxation8,9. Strain rate, rather than afterload, is both necessary and sufficient to modify the relaxation rate8. As interventions to modify the calcium rate have not been proven to substantially improve cardiac relaxation, mechanical intervention may provide new insights into the mechanism and provide a new treatment for diastolic dysfunction.
The protocol to modify the myocardial strain rate described here utilizes an isotonic load-clamp8,9. A strength of the isotonic load-clamp is the quantitative control of the afterload stress. Windkessel-like protocols could be used to further probe changes in afterload, preload, and cardiac work2,6,7. A ramp not controlled by the load-clamp could also be used to better isolate the change in strain from strain rate. Regardless, the afterload itself does not appear to be a strong modifier of the relaxation rate8.
The protocol may also be adapted to approach more physiological conditions for temperature and pacing rate. The current protocol details were used to show the presence of the MCR. Conducting experiments in physiological conditions is generally recommended, depending on the experimental question. However, experiments performed at 37 °C, or at high pacing rates, can more rapidly induce rundown (damage) to the muscle. A solution with improved oxygen carrying capacity may be needed. Further, the data acquisition must be able to sample the length and force fast enough to resolve the rapid twitches and provide feedback control.
The current protocol does not describe the measurement of calcium or the measurement and control of sarcomere lengths. Calcium measurements have been addressed in other protocols11, while sarcomere length measurement can be added with appropriate equipment. Sarcomere length control is not utilized in current MCR studies, because muscle length is the most correlative parameter to the clinical condition19. Further sarcomere length control (vs. muscle length control) would provide specific answers to kinetic questions, but is unlikely to add to translational knowledge due to inter-sarcomere variation and the minimum understanding of sarcomere length changes in vivo.
Three experimental considerations are highlighted here to increase the reproducibility of the data.
First, free-standing cardiac trabeculae may be difficult to find in some animals (unpublished results and communications). While twitching muscles can be found in most rats, a reasonable success rate for obtaining data from trabecula in rats is one in three. Trabecula success may be higher with Brown Norway x Lewis F1 rats, which have also been used historically20 and reported to have more trabeculae (unpublished communications). For mice, success rates are likely to be lower, with less than one in 10 expected for mice from a BL/6 background; however, a higher rate is expected for mice from a FVBN background (unpublished communications and observations).
Second, damage to muscles can reduce output. If the developed forces are less than 10 mN mm-2 at 25 °C and 0.5 Hz pacing, investigators may need to perform troubleshooting to assess if inadvertent stretching or contact between metal forceps and muscle is occurring, if solutions are not properly prepared, or if pacing or experimental equipment is functioning properly. Other protocols utilizing intact trabecula have suggested using Luer-lock syringes as transfer vessels11. While this is possible, especially if the user controls a very slow flow rate or smaller muscle segment, the current protocol utilizes a much larger bore transfer pipette to minimize possible damage. Another step where ischemic damage may occur is during dissection. The aorta should be cannulated and flushed with perfusion solution within 3 min of the first abdominal cut (rat) or cervical dislocation (mouse), similar to limits listed in cardiomyocyte isolation protocols21,22. This minimizes the time when the cardiac tissue is not exposed to the cardioplegia-like perfusion solution. Further, dissections lasting more than 30 min generally do not produce twitching trabecula. Thus, operators should practice rapid but careful dissection to minimize damage. A cross-sectional area above 0.2 mm2 (2 x 10-7 m2) may suffer from core ischemia20.
Third, the way in which muscles are attached to the motor and force transducer should be considered. This protocol currently focuses on hooks and free-standing trabeculae. The sometimes rapid strain rate of the stretch prior to relaxation can cause a muscle to slide if not affixed properly, which is why the current protocol does not use "baskets" to hold the trabecula23,24. Alternate mounting methods (adhesives, clips, etc25,26) can also be considered and validated. The protocol described here utilizes trabeculae and not papillary muscles. The chordae of the papillary muscle induce a series elasticity that can inhibit changes to the MCR9. However, the exact placement of the attachments in the muscle is unlikely to impact the measures, because the trabecula length (and diameter) vary substantially.
A limitation of piercing the muscle ends with hooks is that the mounting point itself can also be damaged. Possible tearing of the affixed muscle tissue with frequent contractions (depending on their strength) may change the length or series elasticity. This rate of tearing is difficult to control. Similarly, damage to the tissue and the hook can be exacerbated during stretching, also potentially causing problems. Visual inspection, and the developed force values remaining >80% of the equilibrated isometric force, should be used to assess whether the preparation is damaged and should be excluded.
Another limitation or consideration affects what experimental questions can be answered by the method. For example, consider the use of 2,3-butanedione monoxime (BDM) in the perfusion solution. BDM is a phosphatase, which may alter the function of the muscle. In addition, the long period of unloading and lack of pacing mean that the latent phosphorylation state has likely changed. Thus, caution should be used if trying to directly assess the muscle contractility of an animal (vs. differences between genotypes or treatments), as the contractile state has likely changed. However, the impact of phosphorylation may be assessed pharmacologically by adding an agonist or antagonist of the pathway.
In summary, MCR provides insight into how relaxation is regulated by muscle movement (strain rate). MCR may help to provide better insight into the diagnosis and monitoring of diastolic disease, along with targets for pharmacological intervention, such as modifying myosin kinetics. The protocol and advice outlined here lay out knowledge developed over several years of trials, and should be applicable to other systems and models of cardiac disease.
Disclosures
None.
Acknowledgements
This work is supported by the National Institutes of Health (1R01HL151738) and the American Heart Association (18TPA34170169).
Materials
| Name | Company | Catalog Number | Comments |
| 18 or 16 gauge blunted needle/canula | for cannulation of rat aorta, use 1mm of PE160 or PE205 tubing as stop | ||
| 2,3-Butanedione Monoxime | Sigma-Aldrich | B0753-25G | |
| 23 gauge blunted needle/canula | for cannulation of mouse aorta, use 1mm of PE50 tubing as stop | ||
| 5 mL syringe | BD Luer-Lock | 309646 | |
| 95% Oxygen/5% CO2 | AirGas | Z02OX9522000043 | |
| Anethesia system | EZ Systems | EZ-SA800 | Can use any appropriate anethesia method/system |
| Bovine Serum Albumin | Fisher BioReagents | BP-1600 | to coat tips of fine forcepts, scissors |
| Calcium Chloride Dihydrate | Fisher Chemical | C79-500 | |
| Containers/dissection dishes | FisherBrand | 08-732-113 | Weigh dishes for creating dissection plates |
| Crile Hemostat | Fine Science Tools | 13005-14 | for mouse gross dissection |
| D-(+)-Glucose | Sigma-Aldrich | G8270-1KG | |
| Data acquisition software | SLControl | ||
| Data acquisition system | MicrostarLabs | DAP5216a | Can use any DAQ. This is a PCI based data acqusition for use with SLControl; must have a PC with a PCI slot |
| Data analysis software | Mathworks | Matlab | Custom Script |
| Dumont #3 Forceps | Fine Science Tools | 11231-30 | 2x for cannulation of aorta |
| Dumont #5 Forceps | Fine Science Tools | 11254-20 | 2x for trabecula isolation |
| Experimental system | Aurora Scientific | 801C | Can use any appropriate experimental chamber with force and length control |
| Fine Scissors, curved | Fine Science Tools | 14061-09 | for removal of heart |
| Gooseneck Piggyback Illuminator | AmScope | LED-6WA | |
| HEPES | Sigma-Aldrich | H3375-250G | |
| Imaging software | IrfanView | ||
| Iris Forceps | World Precision Instruments | 15915 | for removal of heart |
| Isoflurane | VetOne | 502017 | |
| Magnesium Chloride Hexahydrate | Sigma-Aldrich | M2670-100G | |
| Magnesium Sulfate | Sigma-Aldrich | M7506-500G | |
| Mayo Scissors | Fine Science Tools | 14110-15 | for rat gross dissection |
| Metzenbaum Scissors | Fine Science Tools | 14116-14 | for mouse gross dissection |
| Microscope connected camera | Flir | BFS-U3-27S5M-C | Includes acquisition software |
| Microscope/digital imaging system | Olympus | IX-73 | Can use any appropriate microscope. Needed to measure muscle length, cross sectional area |
| Mounting Pin/Needle | BD PrecisionGlide | 305136 | For holding heart to dish. 27 G x 1-1/4 |
| Mounting Pin/Needle | Fine Science Tools | 26000-40 | For holding heart to dish. 0.4mm diameter insect pin (Alt to 27G needle) |
| Oxygen (O2) | AirGas | OX USP300 | |
| Peristaltic Pump | Rainin | Rabbit | Can be any means to create flow in experimental chamber |
| pH and Oxygen sensor | Mettler Toledo | SevenGo pH and DO | |
| Potassium Bicarbonate | Sigma-Aldrich | 237205-100G | |
| Potassium Chloride | Fisher Chemical | P217-500 | |
| Potassium Phosphate Monobasic | Sigma-Aldrich | 795488-500G | |
| Rochester-pean Hemostat | World Precision Instruments | 501708 | for rat gross dissection |
| Silk Suture, Size: 4/0 | Fine Science Tools | 18020-40 | cut to ~1.5 inch pieces, soaked in water |
| Sodium Bicarbonate | Sigma-Aldrich | S6297-250G | |
| Sodium Chloride | Sigma-Aldrich | S9888-1KG | |
| Sodium Hydroxide | Sigma-Aldrich | S8045-500G | |
| Sodium Phosphate Dibasic | Sigma-Aldrich | S7907-100G | |
| Stereomicroscope | AmScope | SM-1TX | |
| Student Vannas Spring Scissors | Fine Science Tools | 91500-09 | for opening of the RV |
| SYLGARD 184 Silicone Elastomer Base | Dow Corning | 3097358-1004 | For creating dissection plates |
| Syringe Holder | Harbor Frieght | Helping Hands 60501 | Can be used as alternate for ring stand |
| Taurine | Sigma-Aldrich | T0625-1KG | |
| Transfer Pipette | FisherBrand | 13-711-7M | cut ~1" from tip to widen bore |
| Vannas Spring Scissors | Fine Science Tools | 15000-00 | for trabecula isolation |
References
- Iribe, G., Helmes, M., Kohl, P. Force-length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load. American Journal of Physiology. Heart and Circulatory Physiology. 292 (3), 1487-1497 (2007).
- Dowrick, J. M., et al. Work-loop contractions reveal that the afterload-dependent time course of cardiac Ca(2+) transients is modulated by preload. Journal of Applied Physiology. 133 (3), 663-675 (2022).
- ter Keurs, H. E., Rijnsburger, W. H., van Heuningen, R., Nagelsmit, M. J. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circulation Research. 46 (5), 703-714 (1980).
- Sonnenblick, E. H. Force-velocity relations in mammalian heart muscle. The American Journal of Physiology. 202, 931-939 (1962).
- Brutsaert, D. L., Rademakers, F. E., Sys, S. U. Triple control of relaxation: implications in cardiac disease. Circulation. 69 (1), 190-196 (1984).
- Taberner, A. J., Han, J. C., Loiselle, D. S., Nielsen, P. M. F. An innovative work-loop calorimeter for in vitro measurement of the mechanics and energetics of working cardiac trabeculae. Journal of Applied Physiology. 111 (6), 1798-1803 (2011).
- De Tombe, P. P., Little, W. C. Inotropic effects of ejection are myocardial properties. Am J Physiol. 266, 1202-1213 (1994).
- Chung, C. S., Hoopes, C. W., Campbell, K. S. Myocardial relaxation is accelerated by fast stretch, not reduced afterload. Journal of Molecular and Cellular Cardiology. 103, 65-73 (2017).
- Schick, B. M., et al. Reduced preload increases Mechanical Control (strain-rate dependence) of Relaxation by modifying myosin kinetics. Archives of Biochemistry and Biophysics. 707, 108909 (2021).
- Parikh, S. S., Zou, S. Z., Tung, L. Contraction and relaxation of isolated cardiac myocytes of the frog under varying mechanical loads. Circulation Research. 72 (2), 297-311 (1993).
- Dowrick, J. M., et al. Simultaneous brightfield, fluorescence, and optical coherence tomographic imaging of contracting cardiac trabeculae ex vivo. Journal of Visualized Experiments. (176), e62799 (2021).
- Wiggers, C. J. Studies on the consecutive phases of the cardiac cycle I. The duration of the consecutive phases of the cardiac cycle and the criteria for their precise determination. American Journal of Physiology-Legacy Content. 56 (3), 415-438 (1921).
- Rosen, B. D., et al. Late systolic onset of regional LV relaxation demonstrated in three-dimensional space by MRI tissue tagging. American Journal of Physiology. Heart and Circulatory Physiology. 287 (4), 1740-1746 (2004).
- Saito, M., et al. The differences in left ventricular torsional behavior between patients with hypertrophic cardiomyopathy and hypertensive heart disease. International Journal of Cardiology. 150 (3), 301-306 (2011).
- Monasky, M. M., Biesiadecki, B. J., Janssen, P. M. L. Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. Journal of Molecular and Cellular Cardiology. 48 (5), 1023-1028 (2010).
- Raff, G. L., Glantz, S. A. Volume loading slows left ventricular isovolumic relaxation rate. Evidence of load-dependent relaxation in the intact dog heart. Circulation Research. 48, 813-824 (1981).
- Matsubara, H., Takaki, M., Yasuhara, S., Araki, J., Suga, H. Logistic time constant of isovolumic relaxation pressure-time curve in the canine left ventricle. Better alternative to exponential time constant. Circulation. 92 (8), 2318-2326 (1995).
- Chung, C. S., Kovacs, S. J. Physical determinants of left ventricular isovolumic pressure decline: model prediction with in vivo validation. American Journal of Physiology. Heart and Circulatory Physiology. 294 (4), 1589-1596 (2008).
- Campbell, K. B., Kirkpatrick, R. D., Tobias, A. H., Taheri, H., Shroff, S. G. Series coupled non-contractile elements are functionally unimportant in the isolated heart. Cardiovascular Research. 28 (2), 242-251 (1994).
- Raman, S., Kelley, M. A., Janssen, P. M. Effect of muscle dimensions on trabecular contractile performance under physiological conditions. Pflugers Archiv: European Journal of Physiology. 451 (5), 625-630 (2006).
- Czeiszperger, T. L., Wang, M. P., Chung, C. S. Membrane stabilizer Poloxamer 188 improves yield of primary isolated rat cardiomyocytes without impairing function. Physiol Rep. 8 (4), 14382 (2020).
- Louch, W. E., Sheehan, K. A., Wolska, B. M. Methods in cardiomyocyte isolation, culture, and gene transfer. Journal of Molecular and Cellular Cardiology. 51 (3), 288-298 (2011).
- Loiselle, D. S., Johnston, C. M., Han, J. C., Nielsen, P. M. F., Taberner, A. J. Muscle heat: a window into the thermodynamics of a molecular machine. American Journal of Physiology. Heart and Circulatory Physiology. 310 (3), 311-325 (2016).
- de Tombe, P. P., ter Keurs, H. E. Force and velocity of sarcomere shortening in trabeculae from rat heart. Effects of temperature. Circulation Research. 66 (5), 1239-1254 (1990).
- Palmer, B. M., Bell, S. P. Preparing excitable cardiac papillary muscle and cardiac slices for functional analyses. Frontiers in Physiology. 13, 817205 (2022).
- Brunello, E., et al. Myosin filament-based regulation of the dynamics of contraction in heart muscle. Proceedings of the National Academy of Sciences. 117 (14), 8177-8186 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved