Method Article
Whole Neonatal Cochlear Explants as an In vitro Model
In This Article
Summary
The current protocol updates previous protocols and incorporates relatively straightforward approaches for culturing high-quality cochlear explants. This provides reliable data acquisition and high-resolution imaging in live and fixed cells. This protocol supports the ongoing trend of studying inner ear cells.
Abstract
Untreated hearing loss imposes significant costs on the global healthcare system and impairs individuals' quality of life. Sensorineural hearing loss is characterized by the cumulative and irreversible loss of sensory hair cells and auditory nerves in the cochlea. Entire and vital cochlear explants are one of the fundamental tools in hearing research to detect hair cell loss and to characterize the molecular mechanisms of the inner ear cells. Many years ago, a protocol for neonatal cochlear isolation was developed, and although it has been modified over time, it still holds potential for improvement.
This paper presents an optimized protocol for isolating and culturing whole neonatal cochlear explants in multi-well culture chambers that enables the study of hair cells and spiral ganglion neuron cells along the entire length of the cochlea. The protocol was tested using cochlear explants from mice and rats. Healthy cochlear explants were obtained to study the interaction between hair cells, spiral ganglion neuron cells, and the surrounding supporting cells.
One of the main advantages of this method is that it simplifies the organ culture steps without compromising the quality of the explants. All three turns of the organ of Corti are attached to the bottom of the chamber, which facilitates in vitro experiments and the comprehensive analysis of the explants. We provide some examples of cochlear images from different experiments with live and fixed explants, demonstrating that the explants retain their structure despite exposure to ototoxic drugs. This optimized protocol can be widely used for the integrative analysis of the mammalian cochlea.
Introduction
Most cases of sensorineural hearing loss are due to the degeneration of sensory hair cells, auditory nerve cells, and/or auditory synapses1. This degenerative process in sensory cells is progressive and usually irreversible, thus resulting in hearing loss2. Therefore, information about the viability of sensory cells and changes in signaling pathways under stress conditions is pivotal for protecting the cells from damage and, thus, loss. The investigation of cochlear explants in culture allows the recapitulation of the tissue complex and the maintenance of a normal cell-cell network, which enables a better description of the signaling processes. To establish experimental models of ototoxicity, the antibiotic gentamicin and the chemotherapeutic agent cisplatin have often been used, because they are known to have ototoxic side effects3.
In vitro culture systems of cochlear explants have been developed and modified over time; however, a description of the stepwise protocol for the culture of entire cochlear explants is often missing in several publications. One of the first video protocols for the primary culture of the murine organ of Corti was published by Parker et al., in which the authors described the steps for the isolation of the sensory epithelium, culturing on glass coverslips, and the electroporation of explants for transfection experiments4. Another protocol using glass coverslips was also previously published, in which the organization of cellular structure in the inner ear was considered5. An alternative protocol using Millicell membrane for the culture of mouse explants of the organ of Corti and vestibular organ has been reported6. These video reports have contributed to the enhancement of the method, but there are still challenges that need to be solved. To address a number of issues arising from the use of glass coverslips and inserts, the present protocol aims to streamline the organ culture steps and culture high-quality organs for reliable data. This is achieved by minimizing the direct handling of the organ during the experimental procedures and avoiding organ transfer before obtaining high-resolution images of the live and fixed cells.
The present protocol updates previously published in vitro culture systems and introduces several optimizations in the isolation of the organ of Corti and transfer to the culture chambers, as well as the integration of a new slide chamber to improve the culture conditions and further analysis. This optimized protocol reduces the risk of damaging the organ, which can occur when using glass coverslips during the medium change or during the transfer of the organ from coverslips or membranes for further analysis4,5,6. The glass coverslips have a better reflective index than the plastic ones; they are, however, fragile and can break more easily. The multi-well chambers used here are attached to a microscope slide, which are well-suited for organ culture and for high-resolution imaging. The transfer of the isolated organs is performed with a spatula, which allows the organ to be brought in the right orientation and slid into the chamber, rather than applying force with a pipette as previously recommended4,5,6.
The poly-D-lysine-coated multi-well chambers, which should contain sufficient medium, facilitate the organ transfer and the proper positioning of the explants without applying adhesive pressure and while avoiding organ overlap, as mentioned previously6. In addition, accidental organ overlapping and uneven structures are resolved using a confocal Z-stack. This protocol has been optimized for various applications, such as mouse and rat explants, explants of the organ of Corti and cochlea, culture in serum-containing and serum-free medium, ototoxic assessments, and general drug response experiments. The cochlear explants are mounted and incubated in chambers with a coverslip bottom, which facilitates the adhesion of the cochlear explants to the chambers for optimal handling during the in vitro experiments, the postprocessing of the explants, and the imaging of live and fixed cochlear explants. The visualization of the entire length of the organ of Corti and the quantification of hair cells are streamlined. In addition, the assessments of the supporting cells, spiral ganglion neuron cells, and neurites are accurate. Therefore, this protocol can be used for a comprehensive analysis of mammalian cochlear cells.
Protocol
All the animal procedures were performed in accordance with the guidelines and regulations of the Animal Welfare Committee of Canton Basel City, Switzerland. Postnatal C57BL/6JR mice, Wistar rats, and STAT1-deficient mice (mixed C57BL/6-129/SvEv)7 aged 3-5 days and of either sex were used for the experiments.
1. Coating the multi-well chambers
- Prepare complete culture medium.
- For organ of Corti explants, prepare medium containing Dulbecco's Modified Eagle's Medium (DMEM), 10% fetal bovine serum (FBS), 25 mM HEPES, and 30 U/mL penicillin.
- For organ of Corti explants and cochlear explants, prepare a medium containing DMEM/F12, 1x N2 supplement, 1x B27 minus antioxidants, and 30 U/mL penicillin.
- Prepare a stock solution of poly-D-lysine by adding 10 mL of cell culture water to 5 mg of poly-D-lysine in a laminar hood. The final concentration of poly-D-lysine is 0.5 mg/mL.
NOTE: Aliquot the remaining stock solution, and store at −20 °C.- Prepare working solutions of poly-D-lysine by diluting the stock solution 1:10 in sterile water.
- Coat an 8-well chamber with 150 µL/well of poly-D-lysine working solution, and incubate for 30 min at room temperature.
NOTE: If a 4-well chamber is used, coat with 300 µL/well of poly-D-lysine working solution. - Aspirate the solution by vacuum or pipetting.
- Wash 2x with 200 µL of sterile water and once with 200 µL of complete culture medium or DMEM.
- Add 150 µL of complete culture medium to each well.
NOTE: If a 4-well chamber is used, add 250 µL/well of complete culture medium. - Place the chamber in the incubator at 37 °C and 5% CO2 for at least 30 min before placing an explant.
2. Dissection of the temporal bone
- Disinfect the surgical table with 70% ethanol, and sterilize all the instruments in a glass microsphere sterilizer.
- Place a sterile 60 mm Petri dish on a bucket containing ice.
- Pour a few milliliters of 1x phosphate-buffered saline (PBS), and keep it on ice.
NOTE: Use 30 U/mL penicillin in 1x PBS to avoid bacterial contamination. - Place a sterile pad on an sterile tray and quickly decapitate the pup animal with operating scissors. Submerge the head in 70% ethanol for 5s, if contamination is a frequent event.
NOTE: This protocol was tested for mice and rats aged P3-P5. Cochlear tissues are more difficult to dissect from older mice and rats, and the survival of explants in culture is limited. - Place the animal head on a sterile pad. Remove the mandible. Lift the skin and peel it back from the skull.
- Hold the skull by placing the forceps in the orbital cavities.
- Carefully cut the skull along the sagittal suture, and then cut in the coronal suture area with a sharp scalpel blade without damaging the cochlea.
NOTE: Avoid applying too much pressure, and avoid forward and backward movements with the scalpel, as this may damage the cochlear bone and, thus, the cochlear duct. - Carefully remove the brain from the two skull halves.
- Transfer the skull halves into a 60 mm Petri dish with the ice-cold PBS prepared in step 2.3.
3. Isolation of the cochlea
- Localize the cochlea in the temporal bone under the microscope. Place the forceps in the superior semicircular canal.
- Loosen the surrounding tissue between the cochlea and the temporal bone using an insulin syringe (mice) or forceps (rats).
- Carefully pull the temporal bone away from the cochlea, keeping the cochlea attached to the vestibule with the temporal bone. Ensure that the cochlea is free from the surrounding tissue before pushing the temporal bone aside.
NOTE: Applying too much force will damage the cochlear duct. - Hold the cochlea in a fixed position, and use the other hand to carefully remove the cartilaginous cochlear capsule. Carefully insert the tips of the forceps into the apex region or between the turns (visible as a white line), and remove the capsule piece by piece. Expose the cochlear duct.
- Carefully place the forceps under the cochlea, and detach it from the vestibular organ and temporal bone.
- Transfer the cochlea into a new 60 mm dish containing ice-cold PBS.
- Adjust the magnification of the microscope to better visualize the explants.
- Follow the next steps for organ of Corti explants:
- Hold the organ at the base with forceps. Gently remove the cochlear duct by grabbing it with forceps at the basal hook region.
- Unwind the cochlear duct from the modiolus without tearing it.
- Carefully remove the spiral ligament with the stria vascularis by holding the organ at the base and pulling them away.
NOTE: Tissue separation can also be achieved by holding the apex region instead of the base region. This can be helpful when dissecting rat organs or older mouse pups (>P5). - Carefully remove the Reissner's membrane by holding the organ at the base and pulling it off piece by piece.
NOTE: This is an optional step as the Reissner's membrane does not interfere with the acquisition of the imaging.
- Follow the next steps for cochlear explants:
- Detach the spiral ganglion from the osseous spiral lamina using an insulin syringe (mice) or forceps (rats).
- Gently unwind the modiolus during the detachment.
NOTE: The explants can be divided into two pieces for better handling. - Grasp the hook region with forceps.
- Carefully remove the spiral ligament with the stria vascularis by pulling it off.
- Carefully remove the Reissner's membrane by holding the organ at the base and pulling it off piece by piece.
NOTE: This step is recommended, because the Reissner's membrane usually folds and covers the neuron filaments and, therefore, might affect the experiments.
4. Culture of cochlear explants
- Transfer the explants from the Petri dish to the multi-well chambers. Lift the explants with the hair cells facing up using a laboratory spatula. Include a few microliters of 1x PBS to prevent the samples from sticking to the spatula.
NOTE: Severely damaged explants will stick to the spatula. - Allow the explants to slide from the spatula into the chamber by gently waving the spatula in the medium. Place one explant per well of an 8-well chamber slide.
NOTE: If the explant sticks to the spatula, hold the spatula in the medium, and use the forceps to detach the explants. Always place the forceps at the inner border of the explant (away from the hair cells). - Check under the microscope that the explants have been transferred in the correct orientation and placed in the center of the wells.
NOTE: Incorrectly oriented explants with hair cells facing downwards tend to show a U-shape upward along their width. Correct their orientation by moving the explants to the corners of the chambers (more medium is available) and direct them to rotate. - Remove 80 µL of the medium using a 100 µL pipette, and discard it.
- Check under the microscope if the hair cells and spiral ganglion neuron cells are visible. If necessary, use forceps to gently push apart some overlapping tissue.
- Remove the rest of the medium, and wait for ~10 s.
- Pipet the medium back. Add one or two drops of the medium next to the explant and the rest of the medium at some distance away from the explant to prevent the explant from detaching.
NOTE: Even if the explants are attached to the chambers, the medium should always be added first by pipetting one to two drops next to the explants. In this way, the explants will not be lifted up when the remaining complete media is added. - Return the chamber to the incubator, and incubate for 2 h to allow the organs to attach firmly to the bottom of the chamber.
- Remove the medium, and carefully add 300 µL of fresh prewarmed complete medium using a 100 µL or 200 µL pipette. Do not use a 1 mL pipette.
NOTE: If a pretreatment is desired, after 2 h of attachment, add 300 µL of complete medium containing the substance of interest. If a 4-well chamber is used, use up to 500 µL/well. - Return the chambers to the incubator.
5. Test of ototoxic agents
- Leave the explants overnight to adapt to the culture conditions and to recover.
- Prepare different concentrations of ototoxic agents to find the appropriate concentration to establish an ototoxic model with approximately 50% hair cell loss. Use between 50 µM and 250 µM for gentamicin and between 40 µM and 320 µM for cisplatin. Prepare the cisplatin solutions fresh, and protect them from light.
- Remove the medium, and carefully add 300 µL of medium containing the desired ototoxic drug.
- Incubate the explants with gentamicin and cisplatin at 37 °C for 24 h to 48 h to determine the hair cell survival.
NOTE: The drug concentrations and exposure time are chosen depending on the purpose of the study. The preservation of the explants was here tested up to 72 h. Do not use serum if planning to perform a long-term culture. - Follow the next section to stain the cochlear cells.
6. Fixation and immunofluorescence
- Discard the medium at the end of the experiment, and immediately wash the explants with 200 µL of prewarmed 1x PBS.
- Fix the explants with 200 µL of 4% paraformaldehyde (PFA) for 15 min under a chemical fume hood.
CAUTION: PFA is a hazardous chemical; read the material safety data sheet (MSDS) before working with PFA for the first time. - Wash the explants twice with 200 µL of 1x PBS. Store the explants in 1x PBS at 4 °C in case the staining procedures need to be postponed.
- Prepare the permeabilization solution consisting of 1x PBS and 1%-5% Triton-X100.
- Discard the 1x PBS, add 200 µL of permeabilization solution, and incubate the explants for 15 min.
- Prepare blocking solution.
- For organ of Corti explants, prepare blocking solution consisting of 1x PBS, 10% normal goat serum (NGS, for goat secondary antibodies, or another serum from the same species as the secondary antibodies). Alternatively, use 1%-5% bovine serum albumin (BSA) if the cells are stained only with phalloidin.
- For cochlear explants, prepare blocking solution consisting of 1x PBS, 10% NGS, and Fab fragment (Fab fragment goat-anti mouse IgG H+L, dilution 1:200) if using mouse primary antibodies (e.g., mouse anti-TuJ1) to stain the spiral ganglion cells.
- Add 200 µL of blocking solution, and incubate the explants for 1 h.
- Discard the blocking solution. To those explants incubated with Fab fragment, add 200 µL of 4% PFA, and incubate for 5 min.
- Wash the explants with 1x PBS for 5 min.
- Prepare an antibody solution consisting of 1x PBS, 5% NGS, and 0.1%-0.25% Triton-X100.
- Dilute the primary antibody in antibody solution-MYO7A (ab3481 at a dilution of 1:500 or MYO7A 138-1 at 1:100)-to label the hair cells and TuJ1 (1:400) to label the spiral ganglion neurons.
NOTE: If the hair cells are only labeled with phalloidin, dilute the phalloidin at 1:150 in 1x PBS, incubate for 40 min to 1 h at room temperature, and proceed to step 6.22. - Include a control for the nonspecific binding of the secondary antibody by omitting the primary antibody.
- Add 170 µL of the antibody solution with the primary antibody to the corresponding well, and incubate overnight at 4 °C with gentle shaking (40-60 rpm).
- Wash the explants 4x for 5 min each with 1x PBS.
- Dilute the secondary antibody in antibody solution (e.g., goat anti-rabbit Alexa Fluor 488 or goat anti-mouse Alexa Fluor 568 IgG at a dilution of 1:500).
- Add 170 µL of the antibody solution with the secondary antibody, and incubate for 1 h at room temperature.
NOTE: From this step on, protect the explants from prolonged light exposure. - Wash the explants 2x for 5 min each with 1x PBS.
- Proceed to the next step for the sequential double labeling of the explants. Incubate with a second primary antibody of interest (e.g., MYO7A for hair cells) overnight at 4 °C with gentle shaking (40-60 rpm). Alternatively, incubate with phalloidin (1:150) for 40 min to 1 h at room temperature, and proceed to step 6.22.
NOTE: Perform sequential labeling if the primary antibodies are from the same host species. Perform phalloidin labeling at the end for multiplex immunofluorescence. - Wash the explants 4x for 5 min each with 1x PBS.
- Dilute the secondary antibody in antibody solution (e.g., goat anti-rabbit Alexa Fluor 488 or goat anti-mouse Alexa Fluor 568 IgG at a dilution of 1:500).
- Add 170 µL of the secondary antibody, and incubate for 1 h at room temperature.
- Wash the explants 2x for 5 min each with 1x PBS.
- Prepare a DAPI stock solution of 1 mg/mL, and store at −20 °C. Dilute the DAPI stock solution 1:10 to prepare working solutions of 0.1 mg/mL, and store at 4 °C.
NOTE: Skip step 23 and go to step 26 if the mounting medium containing DAPI is used. - Dilute the DAPI working solution 1:100 in 1x PBS, and incubate the explants with 200 µL of DAPI solution for 5 min.
- Wash the explants 2x for 5 min each with 1x PBS.
- Remove the 1x PBS as much as possible to allow the bottom of the chamber to dry. Do not let the explant dry.
- Wait for 2-5 s, and add one drop of mounting medium directly onto the explant.
NOTE: The mounting medium on the explant will remain in place due to surface tension. Hardening mounting medium can be used. - Store the chambers at 4° C until imaging.
7. Immunofluorescence of live cells from explants
- Use the isolated explants after overnight incubation.
- Remove the medium, and carefully add 300 µL of medium containing 125 µM cisplatin.
- Incubate the explants for 18 h to measure the mitochondrial superoxide in the live explants.
- Discard the medium at the end of the drug exposure.
- Add 300 µL of a permeable probe to detect the cellular ROS (e.g., 250 nM of mito-hydroethidine) and/or Caspase-3 (e.g., 2 µM DEVD peptide conjugated to a nucleic acid binding dye).
- Incubate at 37 °C for 30 min.
- Wash the explants twice gently with 200 µL of warm Hank's balanced salt solution (HBSS) or an appropiate buffer.
- Image the cells within 2 h with fluorescence excitation at 400 nm and emission detection at 590 nm.
- Discard the medium at the end of the experiment, and immediately wash the explants with 200 µL of prewarmed 1x PBS.
- Fix the explants, and stain the cochlear cells as described above.
8. Visualization by confocal imaging
- Image the explants using a microscope equipped with a spinning disk confocal unit or a confocal microscope equipped with a point-scanning confocal unit.
- Acquire the images using a spinning disk with a 20x air objective (numerical aperture: 0.75) for cell counting. Alternatively, acquire the images using a point-scanning confocal microscope with a 40x air objective (numerical aperture: 0.95) or a 100x oil objective (numerical aperture: 1.45) to visualize and count the synapses or image the stereocilia.
NOTE: Adjust the laser intensity and exposure time for each channel to avoid over- and undersaturation of the images. Apply the same settings to all the explants of the same experiment. - Set up the microscope to capture a 3D image of the entire cochlear explant using a 20x air objective, z-stack, and automatic stitching tools. Use 3 x 3 adjacent fields with 15% overlap for mouse explants and 4 x 4 adjacent fields for rat explants to be stitched together.
- Adjust the images using the microscope's software or the free open-source FIJI software8.
NOTE: Deconvolution, an image processing technique, can be applied to confocal images to sharpen their contrast and resolution9-11.
Results
The present protocol has been tested on the cochlea of neonatal mice and rats. This paper presents images of explants from different experiments. The explants of the organ of Corti were exposed to gentamicin or cisplatin, and hair cell loss was visible. The explants of the organ of Corti maintained their structure and total length under both normal and stress conditions (Figure 1 and Figure 2). The surviving hair cells along the entire length of the rat explants previously exposed to cisplatin were individually detectable (Figure 1). In addition to the detection of surviving hair cells, hair cells undergoing apoptosis were also detected (Figure 2). This approach facilitates the visualization and counting of surviving cells, which can be performed using a deep learning approach, as described previously12. It was also possible to detect the biological processes in living cochlear cells using appropriate cell-permeable probes (Figure 3).
In the case of cochlear explants containing spiral ganglion neurons, the explants can be cut into two pieces, or the apex region can be cut away to provide better culture conditions. Here we chose to separate the apex region, because it is less affected under stress conditions. Figure 4 shows the base and medial regions of the cochlear explants. Hair cells labeled with the hair cell marker MYO7A were detected. Similarly, healthy and damaged spiral ganglion cell bodies and neurites labeled with the neuronal marker TuJ1 were identified. The analysis of spiral ganglion regions can be performed manually or using open-source software such as FIJI with the NeuronJ plugin for neurite tracing13 or extensions such as TrackMate and Cellpose for morphological segmentation14,15. The closer examination of the mouse explants revealed the high resolution of the cochlear cells and hair cell stereocilia (Figure 5).
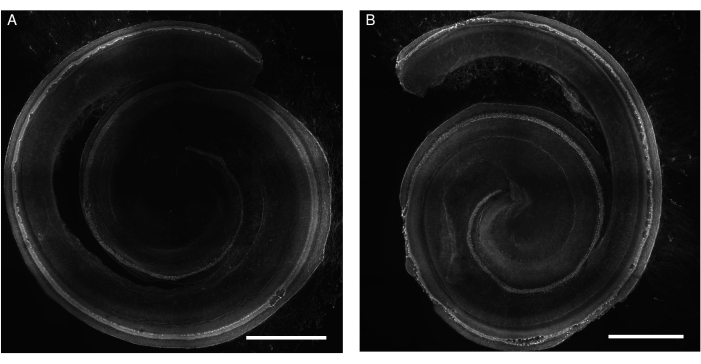
Figure 1: Explants of the organ of Corti from rats exposed to gentamicin. Representative images (maximum intensity projection) of (A) control and (B) gentamicin-exposed (200 µM for 24 h) explants. The hair cells are labeled with phalloidin and can be visualized along the entire length of the cochlea. For better illustration, the image is in gray tones. The images were acquired using a spinning disk confocal microscope with a 20x objective (numerical aperture: 0.75). Scale bar = 500 µm. Please click here to view a larger version of this figure.
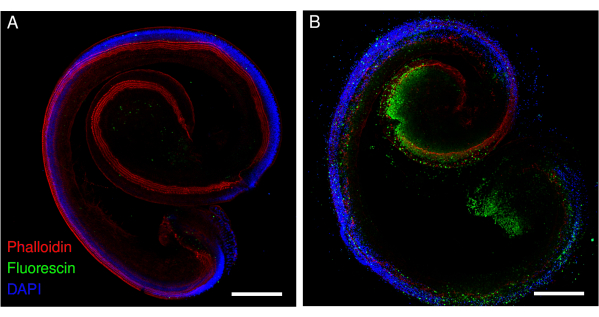
Figure 2: Explants of the organ of Corti from mice exposed to cisplatin. Representative images (maximum intensity projection) of (A) control and (B) cisplatin-exposed (160 µM for 48 h) explants. The hair cells are labeled with phalloidin (red), and the apoptotic hair cells are labeled with fluorescin. The images were acquired using a point-scanning confocal microscope and a 20x objective (numerical aperture: 0.75). Scale bar = 200 µm. Please click here to view a larger version of this figure.
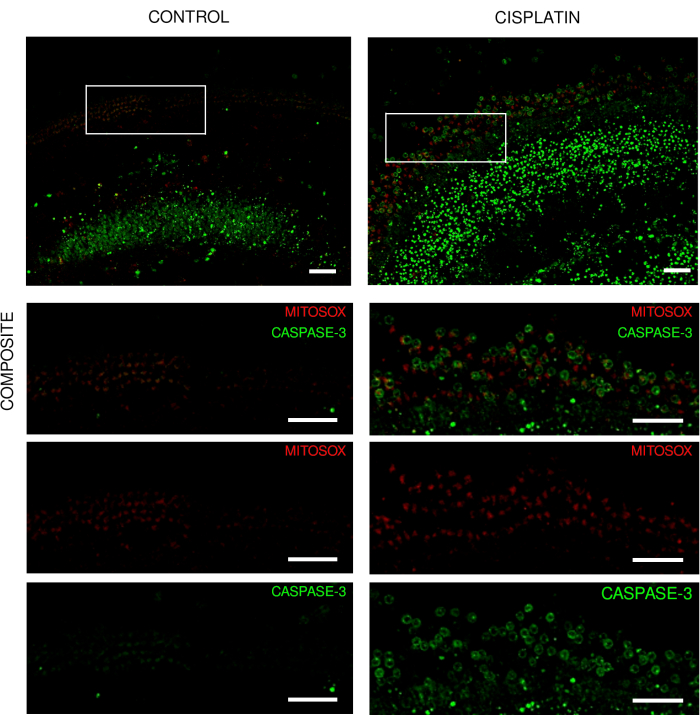
Figure 3: Explants of the organ of Corti from live imaging experiments. Representative images (maximum intensity projection) of explants from wild-type mice showing (A) control explants and (B) explants with exposure to 125 µM cisplatin for 18 h. The hair cells are labeled with mito-hydroethidine and caspase-3. The images were acquired using a spinning disk confocal microscope and a 20x objective (numerical aperture: 0.75). Scale bar = 50 µm. Please click here to view a larger version of this figure.
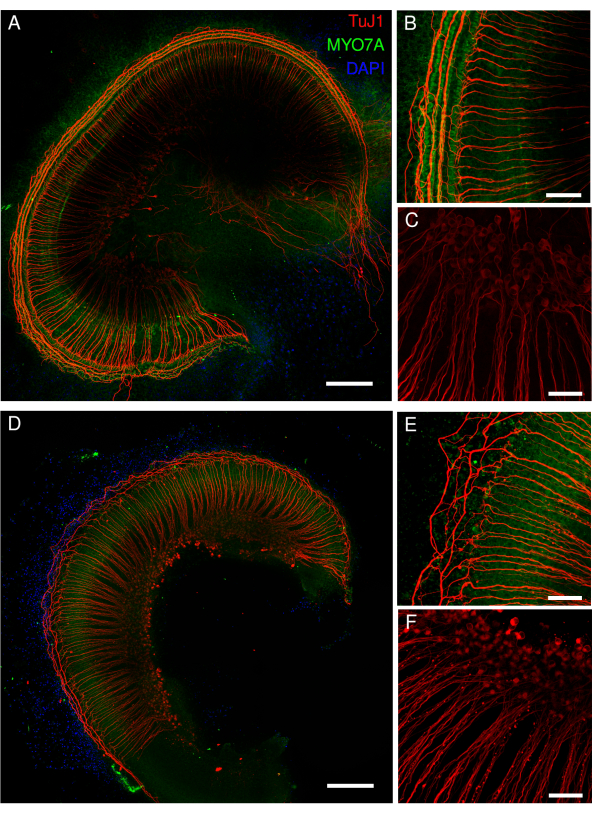
Figure 4: Cochlear explants from STAT1 knockout mice exposed to cisplatin. Representative images (maximum intensity projection) of (A) control explants and (D) explants exposed to 40 µM cisplatin for 48 h. The hair cell bodies are labeled with the MYO7A antibody (green), and the spiral ganglion cells with the TuJ1 antibody (red) and DAPI nuclear labeling (blue). The images were acquired using a point-scanning confocal microscope and a 20x objective (numerical aperture: 0.75) with an additional 2.15 zoom (B,C,E,F). Scale bar = (B,C,E,F) 50 µm and (A,D) 200 µm. Please click here to view a larger version of this figure.
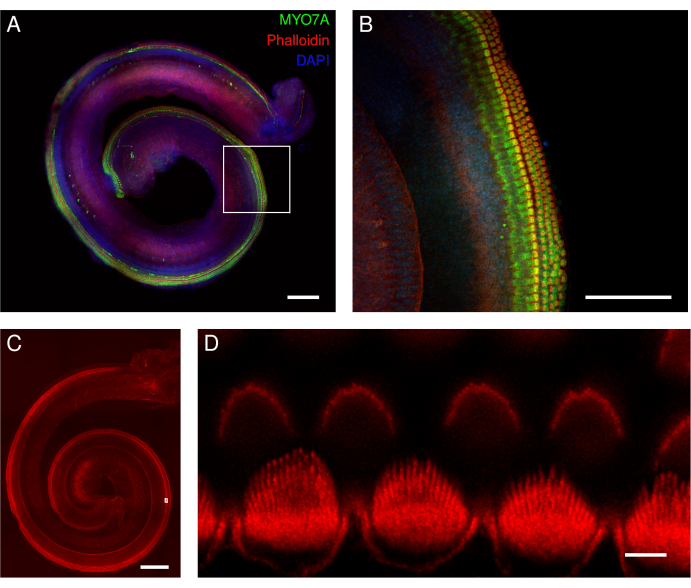
Figure 5: Mouse organ of Corti explants. (A-D) Representative images (maximum intensity projection) of full-length explants from wild-type mice. (A,B) The explants were labeled with the MYO7A antibody, phalloidin, and DAPI nuclear labeling. (B) In the magnified overview, the cell bodies of the hair cells labeled with MYO7A antibody (green) are clearly visible. (C,D) Phalloidin labels the stereocilia and cuticular plate of the hair cells. The explants were labeled with phalloidin. (D) In the magnified overview, deconvoluted images of individual inner hair cell stereocilia are well identified (lower row), whereas individual outer hair cell stereocilia are more difficult to delineate (upper row). The images in panels A and C were acquired with a spinning disk confocal microscope with a 20x objective (numerical aperture: 0.75). The image in panel B was acquired with the same microscope but with a 40x air objective (numerical aperture: 0.95). The image in panel D was acquired with a point-scanning confocal microscope and a 100x oil objective (numerical aperture: 1.45) with an additional 3.46 zoom. The scans were averaged four times per XY section, and the pixel size was 0.02 µm. Scale bars = (D) 3 µm, (B) 100 µm, and (A,C) 200 µm. Please click here to view a larger version of this figure.
Discussion
The purpose of updating this protocol was to streamline the steps from the isolation of the explants to the imaging of the live and fixed cochlear cells. We improved some steps during the isolation and introduced some innovative tools with the aim of establishing an efficient and smooth-running protocol to obtain high-quality explants. The method described is an optimized protocol from previous reports4,5. In addition, some current studies lack a stepwise updated protocol. With simplified explant culture steps, this protocol provides the easy handling of well-preserved explants, which is essential for reproducible data. The introduction of multi-well chambers with a polymer coverslip for inner ear explants improves the organ attachment and the preservation of intact explants. Here, we present several examples of experiments under stress conditions to demonstrate that the organs in culture maintain their cellular organization despite the loss of hair cells and damage to the neurites.
One of the challenges in culturing inner ear organs is avoiding the detachment and floating of the organs, as this affects the integrity of the explants, the response to treatment, and the subsequent examinations. Previously, explants were cultured on glass coverslips4,5. Although culture on glass surfaces seems to be a good alternative, coating the glass is time-consuming, and the coverslips themselves are fragile and delicate. An alternative protocol using Millicell cell culture inserts attempts to resolve this problem6. However, cutting and transferring the membrane with the explants seems to be a delicate step in that protocol. In addition, the explants may be damaged during the mounting and sealing of the coverslip. In our proposed approach, once the explants are transferred into the poly-D-lysine coated chambers and placed in the correct position, no further transfer or covering with coverslips is required. A further advantage of this protocol is the use of chambers with a thin gas-permeable polymer coverslip that provides optimal culture conditions for the organ explants. This polymer has an optical quality similar to glass, thus making it suitable for cell imaging in high-resolution microscopy.
The addition of serum to the medium is used in most protocols for cell and tissue culture, including the culture of inner ear explants with 1%-10% FBS4,5,6,16. The presence of serum affects the culture conditions of the experiments; thus, in certain situations, culture without serum is preferred. The absence of serum in the culture of cochlear explants was replaced either by the addition of N2 to DMEM or by the addition of N2 to Neurobasal-A medium5,6. In this regard, we tested the culture conditions of the explants with and without serum. Under both conditions, the inner ear cells were vital and responded to ototoxic conditions. We tested these conditions for 72 h, but the explants can be maintained in culture for even longer, especially when incubated with serum-free medium together with N2, B27, and growth factors, as suggested in other studies5,16.
In addition to the general critical steps in the isolation of inner ear explants, such as the duration of the organ isolation and the antibiotic used, there are also some critical steps in this protocol, which are, however, manageable. One of the critical steps in this method is related to the volume of medium that remains in the chamber after the organ is inserted. This has been optimized to keep the explants alive and attached to the bottom surface. Another critical step is related to the incubation time required to allow the explants to attach to the bottom of the chamber. Incubation times longer than 2 h with a few microliters of medium could affect the health of the explants. Shorter incubation times, such as 1 h, can also be used, as long as care is taken not to detach the explants. Another important aspect is the residues of poly-D-lysine. The washing steps of poly-D-lysine should be strictly followed, because residues of the bromide salt of poly-D-lysine can be toxic to the cells. After the washing steps have been followed precisely, the coating with poly-D-lysine facilitates the smooth adhesion of the explants to the chambers so that the position can be corrected before they become firmly attached to the bottom of the chamber.
One of the limitations of this method is the imaging of cells using upright microscopy. This could be an important issue for those laboratories with inverted microscopes. Glass slides with removable silicone chambers can be used for upright and inverted microscopy; however, our coating conditions with poly-D-lysine need to be tested first. A further limitation is the storage of the chambers, because the inserts are not removable, and the total height of one chamber with the lid is nearly 11 mm compared to the 1 mm height of a standard microscope slide. However, the 8-well chamber uses less space than the 4-well plates suggested before16.
We present here images acquired with two microscopes. While the point-scanning confocal microscope provides high-resolution images of tissues due to its thin optical section, the spinning disk confocal microscope provides a faster imaging time with good resolution. The stereocilia of the inner hair cells (IHCs) and the outer hair cells (OHCs) are visualized using confocal microscopy. Since the stereocilia of IHCs are larger than those of OHCs, they were repeatedly and well visualized in this work. For OHC stereocilia, other alternative microscopes can improve the visualization, such as super-resolution microscopy (SRM). The explant images acquired with the spinning disk microscope are sufficient for the easy integration of automated hair cell counting using a deep learning approach12. Moreover, the short acquisition time is important for experiments with live cells and tissues. In addition, this protocol is not limited to neonatal cochlear explants. With some optimizations, other explants such as vestibular organs or embryonic tissues can also be cultured.
The quantification of cochlear cells, such as hair cells and neurons, in vitro is important for assessing cell viability and, thus, the percentage of damaged or lost cells. Investigations of signaling pathways and cell functions help to reveal the mechanisms of death and survival. Examinations of embryonic and neonatal cochlear tissues are useful for investigating the developmental stages of the cochlea. Therefore, this protocol will help to optimize in vitro studies of inner ear explants, for example, to establish ototoxic models, investigate developmental stages, evaluate signaling pathways, and perform drug screening studies.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We would like to acknowledge the Animal Facility of the Department of Biomedicine of the University of Basel for their support in animal care, the Microscopy Core Facilities as well as the Information Technology Service of the Department of Biomedicine for their technical assistance, and the Swiss National Science Foundation (SNSF) for financial support (MD-PhD scholarship to M.C., grant number 323530_191222).
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL High-Clarity Polypropylene Conical Tube 17 x 120 mm style | FALCON | 352096 | |
| 45° Angled Forceps | Fine Science Tools | 11251-35 | |
| 50 mL Polypropylene Conical Tube 30 x 115 mm style | FALCON | 352070 | |
| Antifade Mounting Medium | VECTASHIELD | H-1000 | |
| Alexa Fluor 568 phalloidin | Thermofisher | 2151755 | |
| Anti-beta III Tubulin antibody [TUJ-1] | Abcam | ab14545 | |
| Antifade Mounting Medium With DAPI | VECTASHIELD | H-1200 | |
| Anti-myosin VII rabbit polyclonal | Abcam | ab3481 | |
| B-27 Supplement (50x), minus antioxidants | Thermofisher | 10889038 | |
| CARBON STEEL surgical blades 23 | Swann Moiton | 210 | |
| CellEvent™ Caspase-3/7 Green Detection Reagent | Thermofisher | C10723 | |
| DMEM/F-12/(1:1)(1x) + GlutaMAX | Thermofisher | 31331028 | |
| Double spatulas, one curved end | VWR | RSGA038.150 | |
| Ethyl alcohol 70% V/V 1,000 mL | bichsel | 160 0 106 00 | |
| Fetal Bovine Serum, certified | Thermofisher | 16000036 | |
| Fixative Solution 4% paraformaldehyde prepared in PBS | Thermofisher | 201255309/201255305 | |
| High Intensity Cold Halogen Light Source | Intralux® | 5100 | |
| Huygens Professional version 21.10 | Scientific Volume Imaging | ||
| ibidi µ-Slide 8 well | ibidi | 80826 | |
| microscope | LEICA | M80 | |
| microscope | LEICA | MS5 | |
| MitoSOX™ Red Mitochondrial Superoxide Indicator, for live-cell imaging | Thermofisher | M36008 | |
| N2 supplement (100x) | Thermofisher | 17502048 | |
| Nikon Eclipse Ti microscope with a Yokogawa CSU-W1 spinning disk confocal unit, and a Photometrics Prime 95B camera. | NIKON | ||
| Nikon Eclipse Ti microscope with an A1 point-scanning confocal unit | NIKON | ||
| Operating scissors | Fine Science Tools | 14005-16 | |
| Operating scissors | Fine Science Tools | 14088-10 | |
| Operating tweezers | Fine Science Tools | 11008-15 | |
| PBS pH 7.2 (1x), 500mL | Thermofisher | 20012-019 | |
| Penicillin | Sigma-Aldrich | P3032 | |
| POLY-D-LYSINE HYDROBROMIDE MOL WT GT 30 | Sigma-Aldrich | P7405 | |
| Scalpel Handle #4 | Fine Science Tools | 10004-13 | |
| Steri 250 Second sterilizer | Simon Keller AG | 031100 | |
| Sterilizer, desiccant pellets | Simon Keller AG | 31120 | |
| Tissue Culture Dish 60 x 15 mm | FALCON | 353802 | |
| Tissue Culture Dish 60 x 15 mm | FALCON | 353004 | |
| Trito X-100 | Sigma | T9284 | |
| Unconventional myosin-VIIa | Developmental Studies Hybridoma Bank | 138-1s | |
| WFI for Cell Culture[-]Antimicrobial, 500 mL | Thermofisher | A12873-01 |
References
- Mukherjea, D., et al. The design and screening of drugs to prevent acquired sensorineural hearing loss. Expert Opinion on Drug Discovery. 6 (5), 491-505 (2011).
- Takeda, H., Dondzillo, A., Randall, J. A., Gubbels, S. P. Challenges in cell-based therapies for the treatment of hearing loss. Trends in Neurosciences. 41 (11), 823-837 (2018).
- Schacht, J., Talaska, A. E., Rybak, L. P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. The Anatomical Record. 295 (11), 1837-1850 (2012).
- Parker, M., Brugeaud, A., Edge, A. S. Primary culture and plasmid electroporation of the murine organ of Corti. Journal of Visualized Experiments. (36), e1685(2010).
- Landegger, L. D., Dilwali, S., Stankovic, K. M. Neonatal murine cochlear explant technique as an in vitro screening tool in hearing research. Journal of Visualized Experiments. (124), e55704(2017).
- Ogier, J. M., Burt, R. A., Drury, H. R., Lim, R., Nayagam, B. A. Organotypic culture of neonatal murine inner ear explants. Frontiers in Cellular Neuroscience. 13, 170(2019).
- Durbin, J. E., Hackenmiller, R., Simon, M. C., Levy, D. E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 84 (3), 443-450 (1996).
- Schindelin, J., et al. Fiji: An open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Etournay, R., et al. Cochlear outer hair cells undergo an apical circumference remodeling constrained by the hair bundle shape. Development. 137 (8), 1373-1383 (2010).
- MacDonald, G. H., Rubel, E. W. Three-dimensional imaging of the intact mouse cochlea by fluorescent laser scanning confocal microscopy. Hearing Research. 243 (1-2), 1-10 (2008).
- Sibarita, J. B. Deconvolution microscopy. Advances in Biochemical Engineering/Biotechnology. 95, 201-243 (2005).
- Cortada, M., Sauteur, L., Lanz, M., Levano, S., Bodmer, D. A deep learning approach to quantify auditory hair cells. Hearing Research. 409, 108317(2021).
- Meijering, E., et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 58 (2), 167-176 (2004).
- Ershov, D., et al. TrackMate 7: Integrating state-of-the-art segmentation algorithms into tracking pipelines. Nature Methods. 19 (7), 829-832 (2022).
- Stringer, C., Wang, T., Michaelos, M., Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nature Methods. 18 (1), 100-106 (2021).
- Zhang, L. W., Cang, X. H., Chen, Y., Guan, M. X. In vitro culture of mammalian inner ear hair cells. Journal of Zhejiang University of Science B. 20 (2), 170-179 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved