Method Article
Fu's Subcutaneous Needling for Knee Osteoarthritis Pain
* These authors contributed equally
In This Article
Summary
We present a protocol for using Fu's subcutaneous needling for knee osteoarthritis pain, which combines swaying movement and reperfusion approach techniques. This protocol has great potential for future applications in myofascial pain treatment and could enhance Fu's subcutaneous needling (FSN) manipulation skills.
Abstract
Fu's subcutaneous needling (FSN) is a new acupuncture and dry needling technique based on traditional Chinese medicine. It rapidly produces long-lasting effects in soft tissue injuries, particularly in painful musculoskeletal conditions, by providing stimulation primarily in the subcutaneous area. Osteoarthritis (OA) is the most common joint disease in adults worldwide and is often accompanied by a painful syndrome of structural changes in the peripheral joints of the knee. However, the etiology of OA pain is not fully understood, though myofascial trigger points (MTrPs) are commonly found in the lower limb muscles (so-called "tightened muscles") of patients with knee OA.
FSN has been used in many fields for the treatment of acute pain problems and can relieve muscle contraction from MTrPs, thereby improving the local circulation. This study recruited patients with pain from knee OA into an FSN group or a transcutaneous electrical nerve stimulation (TENS) group with three treatment sessions and a follow-up over the course of 2 weeks. The results showed that FSN was effective in treating soft tissue pain around the knee with OA. This study aimed to establish and visualize three key technical indicators during FSN therapy, including the FSN needle insertion point and layer; the frequency and duration of the swaying movement; and the manipulation of the reperfusion approach. These findings have great potential for future applications in myofascial pain treatment, especially for pain management. Following this protocol could enhance FSN skills.
Introduction
With the aging of the world's population, osteoarthritis (OA) has become one of the most common musculoskeletal disorders in the elderly1. OA is a chronic, localized joint disease, and the prevalence of OA varies between joints, with the knee being the most commonly affected joint2. The current global prevalence of degenerative joint disease of the knee, also known as knee OA is ~3.8%; indeed, the prevalence increased from 4.71 million in 2010 to 5.4 million in 2020, and it may possibly increase to 6.4 million by 20353. The diagnosis of knee OA is primarily defined by pathology, radiology, and clinical symptoms4. Most research in the treatment and diagnosis of knee OA has focused on surgical or pharmacological strategies5. However, joint degeneration involves the cartilage and many surrounding tissues, including the meniscus, subchondral bone, synovium, joint capsule, ligaments, and muscles6. Radiographic imaging and clinical symptoms are often used to determine the stage of knee degeneration and are commonly used as the main basis for diagnosis7. Radiographic findings focus on the narrowing of the joint space, the presence of osteophytes, subchondral sclerosis, and cysts8, while clinical signs include pain, stiffness, swelling, or a feeling of pressure9. The radiographic features of OA are often weakly associated with clinical symptoms10. Some researchers have postulated that the muscles have a significant role in the development of degenerative knee OA11. Among them, the skeletal muscle structure and function are thought to be involved in the development and progression of OA disease in the knee12. Many people with knee OA do not wish to undergo surgery, and most knee patients in primary care in particular have a preference for non-surgical treatment13. As a result, the treatment of degenerative knee OA by treating the skeletal muscles has been of increasing interest to clinicians over the past few years.
The non-surgical treatment of knee OA can be quite challenging, with pain and joint stiffness being the main complaints expressed by patients seeking clinical intervention3. A number of conservative approaches to pain management have been tested, including changes to daily activities and various physiotherapy techniques, but the best approach is still under debate14,15. A preliminary study investigated the association between myofascial trigger points (MTrPs), pain, and function in patients with bilateral knee OA and demonstrated that more active MTrPs are associated with greater persistent pain and reduced physical function16. Therefore, the authors hypothesize that MTrP in the lower limb muscles may be an important source of pain and stiffness in patients with knee OA.
Fu's subcutaneous needling (FSN) is an innovative acupuncture therapy based on acupuncture and traditional Chinese medicine models, and it was developed by the traditional Chinese medicine practitioner Zhonghua Fu17. Recent studies have shown that FSN has a positive effect on the treatment of pain control in musculoskeletal diseases, such as lateral epicondylalgia18, low back pain19, and chronic neck pain20, without adverse side effects18,19,20. The theory of affected muscles (so-called pathological "tightened muscles", with one or more MTrPs in the muscle) in FSN suggests that functional changes in muscles are an important cause of pain and dysfunction in knee joints21. The clinical application of FSN over the past 20 years has led to an increasing refinement of the operational technique and clinical theory; however, there are still no reports or video demonstrations on the detailed treatment of pain caused by various muscle disorders, such as knee OA, with respect to the clinical detection of MTrPs, the identification of the FSN insertion area, and the reperfusion approach techniques as standardized clinical trial practices.
To accelerate the standardization of FSN treatment and facilitate the choice of techniques for future FSN-related clinical studies, this study uses a standardized model for the measurement of the MTrP location, the needle insertion point, the number of swaying movements, and the assessment of the reperfusion approach techniques for knee OA, with transcutaneous electrical nerve stimulation (TENS) treatment as the control group. The protocol aims to provide a more complete technical solution for the analysis of FSN therapy to facilitate future studies.
Protocol
The procedures presented below were approved by the Research Ethics Committee of China Medical University & Hospital, Taichung, Taiwan (CMUH107-REC3-027) and registered at the ClinicalTrials.gov Protocol Registration and Results System (registration number NCT04356651). All patients had to provide their written informed consent before participating in this clinical trial. This experimental protocol illustrates a typical FSN manipulation for use in a laboratory or clinical setting.
1. Recruitment of patients with degenerative knee OA
- Use the following inclusion criteria for the screening process: (1) aged over 50 years old, (2) diagnosed with knee OA based on radiographic findings (Kellgren and Lawrence Grading above 2); (3) with the clinical symptoms of knee pain, recruiting only the side with the most severe knee pain, with palpated MTrPs in the quadriceps and gastrocnemius muscles; and (4) a visual analog scale (VAS) score > 5 on the side with the most severe knee pain.
- Use the following exclusion criteria: (1) severe internal medical problems, recent injury, trauma, or pregnancy; (2) history of drug abuse (including excessive alcohol consumption); (3) skin infection, ulcers, or injury on the treated knee; (4) history of knee surgery or joint replacement; (5) diseases of the central nervous system or peripheral neuropathy; (6) cognitive dysfunction or unable to participate in the entire trial; and (7) symptoms modified by medication for knee OA in the past 3 months.
2. Treatment groups
- Randomly assign every participant to the treatment group (FSN treatment) or the control group (TENS treatment).
3. Implementation of the FSN manipulation (Figure 1)
NOTE: Although FSN has its origins in traditional acupuncture, the actual procedure is very different. The procedure of FSN treatment is strictly standardized according to the procedures proposed by the developer of the technique. The main emphasis is on the identification of tightened muscles, the selection of the needle insertion points, the swaying movement, and the reperfusion approach.
- Pretreatment preparation
- Selecting the appropriate treatment position
- Ask the participant to lie on their back with the examined knee straight and the pelvis in a neutral position.
NOTE: FSN needles are thicker than acupuncture or dry needling needles, and the duration of FSN manipulation is much longer. Therefore, choosing the correct posture is more crucial for FSN manipulation than for acupuncture or dry needling; lying prone or on one's side are also appropriate for the treatment of posterior lower limb disorders.
- Ask the participant to lie on their back with the examined knee straight and the pelvis in a neutral position.
- Find and confirm the insertion area.
NOTE: FSN does not require the insertion of needles into acupuncture or Ashi points. In most cases, MTrPs are the cause of painful musculoskeletal problems and the main target of FSN therapy.- Ensure that the insertion area is close to the MTrP for single and small MTrPs and near the extremities for large MTrPs or MTrPs clustered in an area.
- As one of the major functional limitations of knee OA is dysfunction of the quadriceps, gastrocnemius, sartorius, and gracilis and semitendinosus muscles, be sure to examine the active and latent MTrPs of the muscles in the lower limb, and measure some of the pain and functional findings associated with the knee OA.
NOTE: In this study, the quadriceps, gastrocnemius, and pes anserinus tendons were found to have a higher number of active MTrPs and were associated with a higher intensity of ongoing knee pain22.
- Sterilization process
- Before the insertion of the FSN needle, sterilize both the surface of the insertion point and the practitioner's fingers.
- Selecting the appropriate treatment position
- FSN needling method and manipulation
NOTE: The operation is divided into two steps: the first step is to insert the FSN needle, and the second step is to handle the FSN needle (swaying movement).- Needle insertion
- Gently remove the protective sheath of the FSN needle, fix the FSN needle in the fixation slot of an FSN-inserting device designed for FSN needle insertion, and pull the fixing groove back into the locked position (Figure 2A).
- Hold the inserting device, push the device into the desired insertion area to create an indentation at ~15° to the skin, quickly pierce the subcutis, and press the control button with the index finger. Once the FSN needle has popped out and penetrated the skin layer, remove the FSN needle from the fixation slot with the other hand, and remove the needle insertion device (Figure 2B).
- Flatten the needle and carefully push it in until it is fully inserted. When pushing forward, slightly raise the needle tip to see if the skin bulge moves with the needle tip. At this stage, ensure that the hand that is pushing the needle remains relaxed and free of resistance and that the patient is not able to feel any movement under the skin, soreness, swelling, or numbness.
- Once the soft casing pipe has been completely buried under the skin, withdraw the needle core handle by ~3 mm, and turn 90° to the left so that the bulge on the bed of the cannula enters the groove of the needle core handle (Figure 2C).
- Locate the insertion point of the FSN needle at the proximal one-third of the line from the superior border of the patella to the anterior superior iliac spine (Figure 2D). Insert the needle in the direction of the patella until it is completely submerged in the subcutaneous tissue.
NOTE: To confirm that the needle is not extending into the dermis or muscle, the participants must confirm that they are completely pain-free throughout the insertion process. To minimize pain during needle insertion, the insertion point of the needling must also be away from the surface vessels, most of which are veins.
- Swaying movement
NOTE: This is the key procedure of the FSN treatment.- Using the needle entry point, slightly remove the needle holder from the skin, and with the thumb as the fulcrum, keep the index, middle, and ring fingers in a straight line. Keep the middle finger and thumb face to face against the needle, and alternate the index and ring fingers back and forth in a smooth, soft, fan-like swaying movement.The angle of the sector is approximately 60°; perform a total of 45 round trips in 30 s (Figure 3A).
NOTE: With a soft and rhythmic operation, the patient will not experience any soreness, numbness, or pain. One round trip constitutes a side-to-side swaying movement of the needle in the horizontal plane.
- Using the needle entry point, slightly remove the needle holder from the skin, and with the thumb as the fulcrum, keep the index, middle, and ring fingers in a straight line. Keep the middle finger and thumb face to face against the needle, and alternate the index and ring fingers back and forth in a smooth, soft, fan-like swaying movement.The angle of the sector is approximately 60°; perform a total of 45 round trips in 30 s (Figure 3A).
- Needle insertion
- Reperfusion approach technique
NOTE: The movement of the relevant muscle or joint is known as the reperfusion approach technique.- Along with the swaying movement, ask the participant to move the relevant muscles or joints by themselves. For example, as done in this study, ask the participant to perform three cycles for a total of 1 min of a dorsiflexion movement with the sole of the foot (Figure 3B, C), with each cycle consisting of 10 s of continuous movement and 10 s of rest.
- Ask the participants to perform three cycles of sitting down and flexing and extending their knee joints for a total of 1 min (Figure 3D, E), with each cycle consisting of 10 s of continuous movement and 10 s of rest. Ensure that the range of motion in the knee extension/flexion and ankle dorsiflexion is as large as possible under safe conditions and is preferably slow.
- Needle withdrawal
- After the swaying movement and reperfusion approach are completed, remove the FSN needle, and apply a dry cotton ball with adhesive tape to the needle hole to avoid bleeding.
4. Implementation of the TENS manipulation
NOTE: TENS is a non-invasive physiotherapy modality that is commonly used to treat acute and chronic pain caused by a variety of conditions. The procedure for TENS treatment emphasizes the patch position selection, current direction selection, and current frequency adjustment.
- Pretreatment preparation
NOTE: TENS is used to help reduce pain and muscle spasms clinically. The device comes with several sets of electrode wires and end pads. The TENS machine runs on batteries.- Place the small electrode connected to the TENS machine on the skin. The machine sends gentle electrical impulses to the electrodes.
- Positioning the pads
- Switch off the TENS device before attaching the pads to the participant's skin. Place electrodes at the Liangqiu (ST34) and Yanglingquan (GB34) points on the lateral knee and at the Xuehai (SP10) and Yinlingquan (SP9) points on the medial knee (Figure 4).
- Current direction and frequency adjustment
- Turn on the TENS machine after the pads are attached in the correct places. Let the current pass through each patch and across the knee joint, and select a continuous wave (ADJ waveform) with a pulse frequency of 110 Hz and a continual stimulation of 20 min.
- Switch the TENS machine off after using it, and peel off the electrode pads from the participant's skin.
5. Post-intervention and follow-up outcome assessments
NOTE: The entire experiment course lasted 2 weeks. In this trial, a total of three treatment sessions were administered in the first week, with assessments before and immediately after each session, and follow-up visits were conducted in the subsequent weeks 1 and 2. Outcome measurements, which included the pain qualities, the muscle and tendon qualities, and the functional index questionnaire assessment were used.
- Pain qualities
- Assess the patient's subjective pain intensity by asking the patient to fill out a VAS to rate the pain intensity around their knee before and after every treatment.
- Use a physical ability detection system containing a high-speed 3D accelerometer and gyroscope to capture walking speed databefore and after every treatment.
- Place the measurement tool in the area below the shoulder blades, and use a special strap with a comfortable three-button fastening that naturally combines ground measurement information with trunk kinematics to provide accurate information about the stability and coordination of the upper body.
- Allow the patient to walk in a straight line on a horizontal plane for 30 s.
- Record the participant's projected travel speed via a computer connection.
- Use a goniometer to measure the progress in the range-of-motion (ROM), including the active ROM (AROM) and passive ROM (PROM), of the treated knee.
- Perform knee flexion ROM with the patient in the supine position lying on their back on a hard surface.
- Align the goniometer against the participant's leg, ensuring that the circular disc at the center of the goniometer is against the side of the treated knee. Place the fixed arm of the goniometer along the lateral thigh so that it is in line with the end of the greater trochanter or femur attached to the bone at the hip, and place the moving arm of the goniometer along the fibula to the lateral malleolus. Have the clinician grasp the anterior part of the patient's lower leg with one hand, just proximal to the ankle bone, and the anterior part of the patient's thigh with the other hand, just above the greater trochanter major.
- Bend the patient's hip to approximately 90°, and hold it in place with one hand while bending the knee with the other hand; slide the foot up close to the hip. Keep going until the treated knee reaches the maximum point of knee flexion ability. This is how much the knee can bend and straighten on its own, meaning that the knee muscles contract actively without any external help; this is also known as the AROM.
- Apply a slight overpressure to determine how far the knee can be flexed and straightened when moved by an external force, commonly another person; this is called the PROM.
- Muscle and tendon qualities
NOTE: The pressure pain threshold (PPT) is used to measure deep muscular tissue sensitivity, which is a quality of the muscles and tendons. We use an algometer to measure the PPT values of the quadriceps muscle, pes anserinus, and gastrocnemius muscle. According to previous research, the test-retest reliability of the PPT is excellent23.- PPT measurements
- When measuring the PPT of the quadriceps muscle, instruct the participant to lie flat on the physiotherapy couch and maintain a straight-knee position with the skin of the quadriceps muscle exposed. Use an assistant's help or a small, soft cushion to adjust the direction of the center of the knee to face upward.
- When measuring the PPT of the pes anserinus, place the participant in the supine position on the physiotherapy couch, with the knee flexed 5-10° and the skin of the pes anserinus exposed.
- When measuring the PPT of the gastrocnemius muscle, instruct the participant to lie in the prone position with their legs extended, their feet over the end of the physiotherapy couch, and the skin of the gastrocnemius muscle exposed.
- Find the MTrPs or tender points, and mark them on the skin. The MTrP of the quadriceps muscle is located on the rectus femoris muscle and above the patella.
NOTE: The tender point of the pes anserinus measured in this study was located approximately 6 cm distal to the knee joint line along the anteromedial tibial shaft. The MTrP of the gastrocnemius muscle we found and selected for our measurement was in the medial head of the gastrocnemius below the crease of the knee. - Apply a variable pressure of 0.5-1 N/cm2 in the vertical direction relative to the MTrPs on the quadriceps and gastrocnemius muscles and the tender point of the pes anserinus, slowly increasing the force applied.
- The threshold for a latent MTrP is indicated when the patient starts to feel pain or discomfort, and the threshold for an active MTrP is indicated when the pain intensity continues to increase to the point of intolerability. When the participant feels that the pain is intolerable and says "stop", record the data on the stress pain meter, and ask the participant to remember the level of pain or discomfort before proceeding to the next test. Reset the device to zero before the next test. Ensure that the examiner does not see the score displayed when the pressure is applied; test the point three times.
- Provide a 60 s rest interval between trials. The mean of the values after three measurements is defined as the PPT of the quadriceps muscle, the pes anserinus, and the gastrocnemius muscle.
- PPT measurements
- Functional index questionnaire assessment
- Administer the Western Ontario and McMaster Universities Arthritis Index (WOMAC) test using the standardized questionnaire.
NOTE: The WOMAC was developed in 1982 at Western Ontario and McMaster Universities24. In the WOMAC, the participants are asked to complete a standardized questionnaire. The self-administered questionnaire consists of 24 items divided into three subscales that measure three separate dimensions, including pain (5 questions), stiffness (2 questions), and function (17 questions). These items are scored from 0 to 4, which correspond to none (0), mild (1), moderate (2), severe (3), and extreme (4), and the maximum score is 96. Higher WOMAC scores indicate worse pain, stiffness, and functional limitations. - Determine the Lequesne index by using the standardized test and asking the participants to complete the standardized questionnaire.
NOTE: The Lequesne index is divided into three main components for self-assessment related to daily routines. The first is a pain and discomfort score ranging from 1 to 8; higher scores indicate more painful clinical symptoms. The second component is the walking distance score, which ranges from 1 to 8; higher scores indicate greater difficulty in walking. The third component is the daily joint mobility score, which ranges from 0 to 8. The total score ranges from 1 to 24, with higher scores indicating more severe clinical symptoms, such as more severe degeneration and inflammation of the joint.
- Administer the Western Ontario and McMaster Universities Arthritis Index (WOMAC) test using the standardized questionnaire.
6. Statistics
- Present the data as mean ± standard deviation. Analyze the baseline characteristics of age, sex, height, weight, VAS, WOMAC, Lequesne index, PPT, ROM, and walking speed using a Student's t-test. The continuous variables are the VAS, PPT, walking speed, ROM, WOMAC, and Lequesne Index scores. Set the significance level at p < 0.05.
- The immediate effect is the variable change occurring immediately after each treatment. Identify the 1 week effect by comparing the variables at 1 week after treatment with their values before the first treatment. Determine the 2 week effect by comparing the variables at 2 weeks after treatment with their values before the first treatment.
- Perform a comparison between the groups before and after intervention using an independent samples t-test.
Results
The described protocol was implemented in a clinical setting at the China Medical University Hospital of Taiwan, and its feasibility and outcomes were assessed in a recently published clinical study25. The study enrolled a total of 31 participants (10 males, 21 females) to complete the intervention. The FSN group consisted of 15 participants (4 males, 11 females, mean age: 65.73 years ± 6.79 years), while the TENS group consisted of 16 participants (6 males, 10 females, mean age: 62.81 years ± 5.72 years) (Table 1). The results of the study showed that the FSN group exhibited a significant improvement in pain characteristics as measured by the VAS (p < 0.05) (Table 2). The study also revealed a significant difference in the PPT of the quadriceps muscle in the FSN group (p < 0.05), indicating an improvement in the muscle and tendon qualities, and this was particularly noticeable among the participants who received immediate treatment (Table 3). The functional index questionnaire assessment revealed that the FSN group demonstrated significant enhancements in the WOMAC and Lequesne index scores, reflecting improvements in physical function, pain, and stiffness. The improvements were noticeable in the immediate, 1 week, and 2 week follow-up periods (p < 0.05) (Table 4). The findings of this study provide evidence to support the feasibility of FSN therapy as a treatment option for patients suffering from painful knee OA. The results also establish the effectiveness of FSN treatment in alleviating the soft-tissue pain associated with knee OA caused by MTrPs (Figure 5).
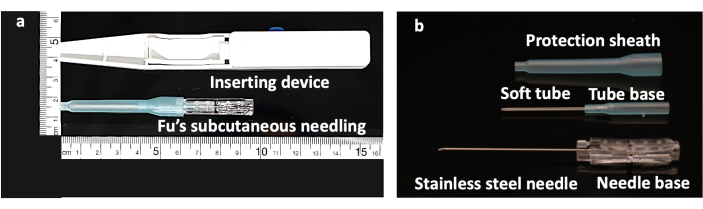
Figure 1: Structure of the Fu's subcutaneous needling needle. (A) FSN-inserting device with a FSN needle. (B) The FSN needle is made up of three parts: a solid steel needle core with a needle base (bottom), a soft tube (middle), and a protection sheath (top). Abbreviation: FSN = Fu's subcutaneous needling. Please click here to view a larger version of this figure.
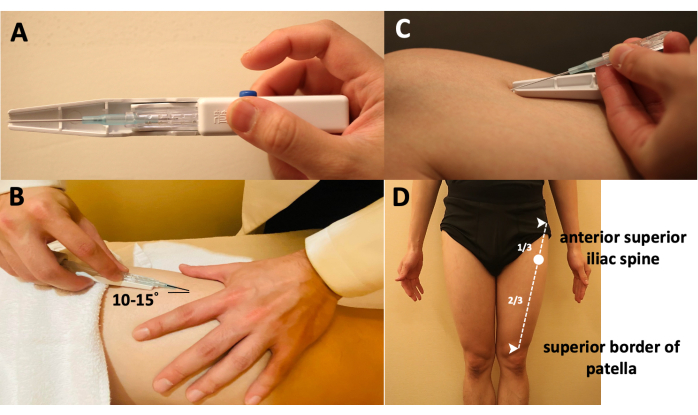
Figure 2: Manipulations of the Fu's subcutaneous needling needle. (A) The way of holding the inserting device. (B) The method to insert the FSN needle into the skin-the needle tip is placed at approximately 15° to the skin. (C) The method to separate the FSN needle from the inserting device. (D) Locating the insertion point, which is at the proximal one-third of the line from the anterior superior iliac spine to the superior border of the patella. Abbreviation: FSN = Fu's subcutaneous needling. Please click here to view a larger version of this figure.
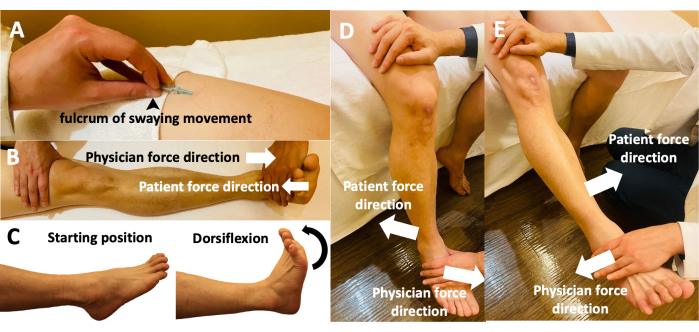
Figure 3: Fu's subcutaneous needling manipulations of the participants' limbs. (A) The holding of the FSN needle while performing the swaying movement. Using the thumb as the fulcrum, the middle finger and thumb affix the needle in a face-to-face manner, with the index and ring fingers moving back and forth. (B) Reperfusion approach with the participant performing a dorsiflexion movement and the physician performing an antagonistic movement with opposing dorsiflexion forces. (C) Reperfusion approach with the participant actively moving the relevant muscles and joints during dorsiflexion from the starting position. (D) Reperfusion approach with the participant actively performing knee flexion with the physician's resistance. (E) Reperfusion approach with the participant performing active knee extension against the physician's resistance. Abbreviation: FSN = Fu's subcutaneous needling. Please click here to view a larger version of this figure.
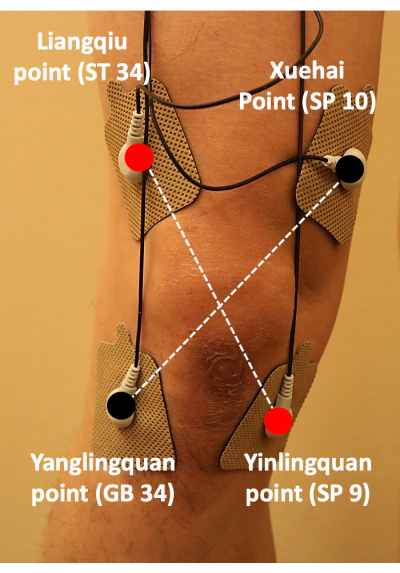
Figure 4: Positioning of the transcutaneous electrical nerve stimulation pads. TENS pads were attached at ST34, GB34, SP10, and SP9; the pads were placed in a cross pattern to treat the pain associated with knee osteoarthritis. Abbreviation: TENS = transcutaneous electrical nerve stimulation. Please click here to view a larger version of this figure.
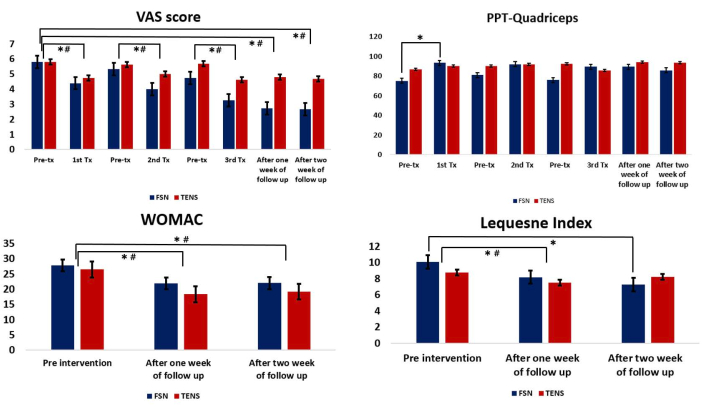
Figure 5: Comparison between the Fu's subcutaneous needling and transcutaneous electrical nerve stimulation groups. (A) The pre- and post-treatment values of the VAS. (B) The pre- and post-treatment values of the PPT for the quadriceps muscle. (C) Comparison of the WOMAC between the two groups after each treatment. (D) Comparison of the Lequesne index between the two groups after each treatment. * Represents the FSN group, p < 0.05; # represents the TENS group, p < 0.05. Abbreviations: VAS = visual analog scale; PPT = pressure pain threshold; WOMAC = Western Ontario and McMaster Universities Arthritis Index; Tx = treatment; FSN = Fu's subcutaneous needling; TENS = transcutaneous electrical nerve stimulation. Please click here to view a larger version of this figure.
Table 1: Baseline characteristics and clinical evaluation indicators of the participants. Data are expressed as mean ± SD; the P values were obtained from analyses with independent samples t-tests. This table is from Chiu et al.25. Abbreviations: FSN = Fu's subcutaneous needling; TENS = transcutaneous electrical nerve stimulation; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; PPT = pain pressure threshold; ROM = range of motion. Please click here to download this Table.
Table 2: Pain qualities compared between the FSN and TENS groups. Data are expressed as mean ± SD. This table is from Chiu et al.25. Abbreviations: FNS = Fu's subcutaneous needling; TENS = transcutaneous electrical nerve stimulations; VAS = visual analog scale; tx = treatment; F/U = follow-up. * Indicates a significant difference, as analyzed by a paired t-test. Please click here to download this Table.
Table 3: Muscle and tendon qualities (PPT of the quadriceps muscle) compared between the FSN and TENS groups. Data are expressed as mean ± SD. This table is from Chiu et al.25. Abbreviations: FNS = Fu's subcutaneous needling; TENS = transcutaneous electrical nerve stimulations; PPT = pain pressure threshold; tx = treatment; F/U = follow-up. * Indicates a significant difference, as analyzed by a paired t-test. Please click here to download this Table.
Table 4: WOMAC and Lequesne index compared between the FSN and TENS groups. Data are expressed as mean ± SD. This table is from Chiu et al.25. Abbreviations: FNS = Fu's subcutaneous needling; TENS = transcutaneous electrical nerve stimulations; WOMAC = Western Ontario and McMaster Universities Arthritis Index; tx = treatment; F/U = follow-up. * Indicates a significant difference, as analyzed by a paired t-test. Please click here to download this Table.
Discussion
The main findings of this study are as follows: (1) confirmation of the approach and complete procedure of the FSN treatment of knee OA; and (2) assessment of the improvement from before to after FSN treatment using a standardized assessment approach. Unlike traditional acupuncture and dry needling, FSN requires different forms of movement for clinical treatment, such as swaying movement and the reperfusion approach. The presence of multiple MTrPs, particularly the active and latent MTrPs, can often be a problem for new practitioners in choosing where to insert the needle. In addition, the evaluation of the post-treatment efficacy is also a major problem for FSN therapy, as in the past, it was mostly limited to the subjective descriptions of patients without objective data to evaluate the methods and practices. For these reasons, it has been difficult to standardize the use of FSN in the treatment of disease.
This is the first protocol to use the full procedure of treating degenerative knee OA with FSN and to define a protocol for assessing the improvement in the knee joint from before to after treatment. Knee joint kinematics are complex, as they comprise six degrees of freedom, including flexion/extension, adduction/abduction, and internal/external rotation; therefore, degeneration of the knee joint can seriously affect daily activities26,27. There is growing recognition that improving the health of skeletal muscles can have significant benefits for people with knee OA. Previous studies have shown that pain relief is the main benefit of FSN19, and the most significant and positive correlates of FSN therapy are pain inhibition and increased joint mobility.
FSN therapy has a unique approach; ignoring these differences between FSN and traditional acupuncture can compromise the effectiveness of the treatment. The needling insertion points of FSN are very different from the acupuncture points of traditional acupuncture. The insertion point in FSN is chosen based on a search for the corresponding tightened muscle based on pain (with one or more MTrPs in the muscle) after the treatment area has been determined. Throughout the experiment, there are a number of key steps that affect the results of the analysis. The most important treatment choice in FSN therapy is the selection of the tightened muscle; indeed, MTrPs are considered as a potential new target for therapeutic interventions aimed at treating idiopathic knee OA28. Travell and Simons identified the rectus femoris, vastus medialis, and vastus lateralis muscles as possible sources of MTrPs in people with knee OA29. Henry et al.30 evaluated myofascial pain in total knee replacement patients and concluded that the gastrocnemius and medial femoral muscles had the most MTrPs in their study. In this study, we pre-assessed three muscle segments: the quadriceps muscle, pes anserinus, and gastrocnemius muscle, with the quadriceps muscle being the final muscle chosen as the FSN insertion area. Our selection of the tightened muscle for treatment was similar to that in previous studies, as weakness in the quadriceps is often considered to be the cause of knee OA and is one of the earliest and most common findings in patients with knee OA31. Previous studies have reported that the sensation of knee pain is associated with weakness in the strength of the quadriceps, as muscle control is related to proprioceptive function32,33. Therefore, using FSN to treat the quadriceps in patients with degenerative knee OA could be a clinical priority in the future.
The FSN technique emphasizes the need to avoid soreness, numbness, and pain at the angle of insertion, which is important in order to avoid needle penetration of the vessel wall. In addition, the swaying movement is an important needle technique in FSN therapy, which involves traction on the subcutaneous tissue. The standardized definition of this technique in this paper makes it clearer and simpler for beginners to perform FSN therapy. The reperfusion approach is a complementary method in the process of FSN operation. In FSN therapy, the action of reperfusion forces the affected muscle to contract centripetally or centrifugally so that the local or peripheral arterial pressure of the tightened muscle increases, followed by rapidly stretching the tightened muscle. The reperfusion approach technique is usually used while the clinician performs the swaying movement with the right hand and uses the left hand to facilitate the localized movement of the patient's limbs or uses the left hand or other body parts to facilitate the rhythmic movement of the relevant muscle that is contracting. Although the efficacy of the FSN can be rapidly increased and its adaptability to the specific disease enhanced when the reperfusion approach technique and swaying movement are used simultaneously, this makes the operator's handling of the process more difficult. Through this video protocol, we help students and young practitioners improve their performance of the complex hand movements required for FSN manipulation. Through simple and efficient preparation, a standardized FSN practice can be followed.
The development of this method opens up a new standardized definition of FSN therapy for the treatment of various muscle disorders, and the protocol is considered to be feasible, acceptable, and safe. In the future, the standardized procedure can be employed to provide more data for clinical applications, education, and the application of this procedure to other pain-related disorders and can be used to provide visualized motor learning in FSN education and clinical trials.
Disclosures
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was supported by a grant from the China Medical University Hospital (DMR-109-095) and Asia University Hospital (10951025).
Materials
| Name | Company | Catalog Number | Comments |
| Fu’s subcutaneous needling | Nanjing Paifu Medical Science and Technology Co. | FSN needles are designed for single use. The FSN needle is made up of three parts: a solid steel needle core (bottom), a soft casing pipe (middle), and a protecting sheath (top). | |
| Tissue Hardness Meter/Algometer Combo | ITO Co. | OE-220 | Uses a dedicated measuring device to convert muscle force into a numerical value. Allows objective evaluations of muscle force and eliminates problems of subjective assessments. |
| Transcutaneous Electrical Nerve Stimulation | Well-Life Healthcare Co. | Model Number 2205A | Digital unit which offers TENS. Supplied complete with patient leads, self-adhesive electrodes, 3 AAA batteries and instructions in a soft carry bag. Interval ON time 1 - 30 s. Interval OFF time 1 - 30 s. |
References
- Jang, S., Lee, K., Ju, J. H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. International Journal of Molecular Sciences. 22 (5), 2619 (2021).
- Hunter, D. J., Bierma-Zeinstra, S. Osteoarthritis. Lancet. (10182), 1745-1759 (2019).
- Cross, M., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases. 73 (7), 1323-1330 (2014).
- Liu, Y., Zhang, Z., Li, T., Xu, H., Zhang, H. Senescence in osteoarthritis: from mechanism to potential treatment. Arthritis Research and Therapy. 24 (1), 174 (2022).
- Aweid, O., Haider, Z., Saed, A., Kalairajah, Y. Treatment modalities for hip and knee osteoarthritis: A systematic review of safety. Journal of Orthopaedic Surgery. 26 (3), 2309499018808669 (2018).
- Litwic, A., Edwards, M. H., Dennison, E. M., Cooper, C. Epidemiology and burden of osteoarthritis. British Medical Bulletin. 105, 185-199 (2013).
- Runhaar, J., Kloppenburg, M., Boers, M., Bijlsma, J. W. J., Bierma-Zeinstra, S. M. A. the CREDO expert group. Towards developing diagnostic criteria for early knee osteoarthritis: Data from the CHECK study. Rheumatology. 60 (5), 2448-2455 (2021).
- Altman, R. D., Gold, G. E. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 15, A1-A56 (2007).
- Michael, J. W., Schlüter-Brust, K. U., Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Deutsches Arzteblatt International. 107 (9), 152-162 (2010).
- Bedson, J., Croft, P. R. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskeletal Disorders. 2 (9), 116 (2008).
- Veronese, N., et al. Lower limb muscle strength and muscle mass are associated with incident symptomatic knee osteoarthritis: A longitudinal cohort study. Frontiers in Endocrinology. 16 (12), 804560 (2021).
- Culvenor, A. G., Ruhdorfer, A., Juhl, C., Eckstein, F., Øiestad, B. E. Knee extensor strength and risk of structural, symptomatic, and functional decline in knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care and Research. 69 (5), 649-658 (2017).
- Hawker, G. A., et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients' preferences. Medical Care. 39 (3), 206-216 (2001).
- Braghin, R. M. B., Libardi, E. C., Junqueira, C., Nogueira-Barbosa, M. H., de Abreu, D. C. C. Exercise on balance and function for knee osteoarthritis: A randomized controlled trial. Journal of Bodywork and Movement Therapies. 22 (1), 76-82 (2018).
- Bannuru, R. R., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 27 (11), 1578-1589 (2019).
- Kordi Yoosefinejad, A., et al. Comparison of the prevalence of myofascial trigger points of muscles acting on knee between patients with moderate degree of knee osteoarthritis and healthy matched people. Journal of Bodywork and Movement Therapies. 25, 113-118 (2021).
- Fu, Z. H. . The Foundation of Fu's Subcutaneous Needling. , (2016).
- Huang, C. H., Lin, C. Y., Sun, M. F., Fu, Z., Chou, L. W. Efficacy of Fu's subcutaneous needling on myofascial trigger points for lateral epicondylalgia: A randomized control trial. Evidence-Based Complementary and Alternative Medicine. , 5951327 (2022).
- Ma, K. L., et al. Fu's subcutaneous needling versus massage for chronic non-specific low-back pain: a randomized controlled clinical trial. Annals of Palliative Medicine. 10 (11), 11785-11797 (2021).
- Huang, C. H., et al. Rapid improvement in neck disability, mobility, and sleep quality with chronic neck pain treated by Fu's subcutaneous needling: A randomized control study. Pain Research and Management. 30, 7592873 (2022).
- Fu, Z., Chou, L. W., Dommerholt, J., Fernández-de-las-Peñas, C. Chapter 16 Fu's subcutaneous needling. Trigger Point Dry Needling: An Evidence and Clinical-Based Approach, 2nd edition. , 229-249 (2018).
- Sánchez Romero, E. A., et al. Prevalence of myofascial trigger points in patients with mild to moderate painful knee osteoarthritis: A secondary analysis. Journal of Clinical Medicine. 9 (8), 2561 (2020).
- Wylde, V., Palmer, S., Learmonth, I. D., Dieppe, P. Test-retest reliability of quantitative sensory testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage. 19 (6), 655-658 (2011).
- Chiu, P. E., et al. Efficacy of Fu's subcutaneous needling in treating soft tissue pain of knee osteoarthritis: A randomized clinical trial. Journal of Clinical Medicine. 11 (23), 7184 (2022).
- Markolf, K. L., Yang, P. R., Joshi, N. B., Petrigliano, F. A., McAllister, D. R. In vitro determination of the passive knee flexion axis: Effects of axis alignment on coupled tibiofemoral motions. Medical Engineering and Physics. 67, 73-77 (2019).
- Ghazwan, A., Wilson, C., Holt, C. A., Whatling, G. M. Knee osteoarthritis alters peri-articular knee muscle strategies during gait. PLoS One. 17 (1), e026798 (2022).
- Nguyen, B. M. Myofascial trigger point, falls in the elderly, idiopathic knee pain and osteoarthritis: An alternative concept. Medical Hypotheses. 80 (6), 806-809 (2013).
- Simons, D. G., Travell, J. G., Simons, L. S. . Myofascial Pain and Dysfunction: Upper Half of Body. , (1999).
- Henry, R., et al. Myofascial pain in patients waitlisted for total knee arthroplasty. Pain Research and Management. 17 (5), 321-327 (2012).
- Roos, E. M., Herzog, W., Block, J. A., Bennell, K. L. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nature Reviews Rheumatology. 7 (1), 57-63 (2011).
- Kim, D., Park, G., Kuo, L. T., Park, W. The effects of pain on quadriceps strength, joint proprioception and dynamic balance among women aged 65 to 75 years with knee osteoarthritis. BMC Geriatrics. 18 (1), 245 (2018).
- Lin, J. H., et al. Sensing acidosis: Nociception or sngception. Journal of Biomedical Science. 25 (1), 85 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved