Method Article
Laparoscopic Anterior Right Hepatectomy: A Single-Center Experience
In This Article
Summary
Here we present a step-by-step protocol for conducting laparoscopic anterior right hepatectomy and compare its clinical effects and postoperative outcomes with those of conventional hepatectomies. Analysis of the data of 82 patients with hepatocellular carcinoma revealed that laparoscopic anterior right hepatectomy had better clinical outcomes and survival rates than the conventional hepatectomy.
Abstract
Laparoscopic anterior right hepatectomy (LARH) has been used in some hospitals. However, data on the feasibility and safety of this procedure are still limited, due to the demanding technical requirements. The primary objective of this study was to compare the clinical outcomes of LARH with those of laparoscopic conventional right hepatectomy (LCRH) in patients with large right hepatocellular carcinoma, as well as to confirm the safety and feasibility of LARH. Furthermore, the article presents a step-by-step description of the surgical procedures for LARH to help perform this surgery in the clinic. The principle of LARH is to first prioritize the hepatic inlet duct separation while separating the right hepatic perihepatic ligament after transecting the liver. From December 2015 to June 2022, 82 patients with large right hepatocellular carcinoma (maximum tumor diameter ≥ 5 cm), were recruited for the study. In this cohort, 54 and 28 patients underwent LARH and LCRH, respectively. The perioperative clinical data and survival outcomes of the two groups were compared. Compared with LCRH, LARH exhibited the advantages of less contact and extrusion, thereby leading to the achievement of superior results. Thus, we propose that LARH is the optimal choice for patients with large right hepatocellular carcinoma.
Introduction
Surgery is considered the most effective method to improve the prognosis in patients with hepatocellular carcinoma. However, the right hepatectomy is a difficult procedure. Compared to the conventional approach for right hepatectomy (CA-RH), the anterior approach for right hepatectomy (AA-RH) can achieve better short and long-term effects. Lo et al. reported that AA-RH involves extensive resection (p < 0.001) and concluded that the anterior approach allowed for better mobilization and easier removal of large tumors following liver dissection1. Beppu et al. have reported that AA-RH with hanging maneuver resulted in better overall survival (OS) rates than CA-RH (p = 0.021), because of decreased intraoperative blood loss (p < 0.001) leading to low transfusion rates (p < 0.001)2. In their latest meta-analysis, Jiang et al. analyzed 2297 patients enrolled in 16 studies and confirmed that AA-RH led to faster postoperative recovery and better survival outcomes than CA-RH3.
In 1996, Lai et al. were the first to demonstrate the efficacy and safety of AA-RH through a prospective analysis and compare intraoperative and postoperative outcomes between AA-RH and CA-RH4. Right hepatectomy involves the complete transection of the inflow and outflow vessels of the right liver, in addition to the transection of the liver parenchyma. The mobilization time for the right hepatic lobe varies according to different approaches. Belghiti et al. were the first to propose a hanging maneuver for AA-RH, where the anatomical characteristics of the posterior and inferior hepatic spaces are used5. The liver sling used in their hanging maneuver was a clamped tape that passes behind the liver and around the hepatic parenchyma, elevating the liver away from the anterior surface of the inferior vena cava (IVC). In 2012, Troisi et al. explored laparoscopic AA-RH using a special device called the "Goldfinger dissector" and proposed that it could replace the role of the liver sling in open surgery6. In 2016, Cai et al. adopted the "Goldfinger dissector" hanging maneuver technique through the retro hepatic tunnel7. Since then, this procedure has been gradually accepted in China.
Recently, the development of laparoscopic surgery has promoted the development of laparoscopic anterior right hepatectomy (LARH), a surgical technique involving the combination of AA-RH and laparoscopy. And LCRH involves a combination of CA-RH and laparoscopy. In a prior study, Liu et al. used propensity score matching to show that intraoperative blood loss (p = 0.049) and overall complication rates (p = 0.028) were lower in LARH than LCRH8. The anterior approach is based on the principles of "non-contact and non-extrusion," according to the "no tumor" principle for improved patient survival3. The principle of "non-contact and non-extrusion"aims to avoidprolonged rotation and displacement of the hepatic lobes, to avoid impairment of the afferent and efferent circulation. Furthermore, applying this principle can reduce the probability of tumor rupture, improving the survival prognosis of patients and reducing the risk of impaired liver function caused by liver mobilization.
In LARH, the hepatic inlet duct is dissected first, and the right hepatic perihepatic ligament is transected after liver transection. Additionally, because of the reticular space between the liver and vein, the hanging maneuver can be conveniently performed to guide the resection path correctly. This procedure can further reduce the difficulty in mobilizing the right liver and significantly increase the resection rate of large hepatocellular carcinoma of the right liver. However, LARH has a long operation time and high technical requirements, and the number of reported cases is currently low6,7. Here, we conducted a retrospective study to evaluate the feasibility and safety of LARH, with our findings confirming the strength of this procedure. Furthermore, we compared the clinical outcomes of the two approaches under the premise of the laparoscopic technique to better maintain baseline balance and exclude confounding factors. Therefore, we recommend this procedure for patients with right liver cancer (maximum tumor diameter ≥ 5 cm) for increased resection rate and improved clinical effects.
To demonstrate the step-by-step procedure, we report the case of a 44-year-old woman with an incidentally detected hepatic mass during abdominal ultrasonography. Physical examination revealed no significant abnormalities. Laboratory test results, including routine blood testing, coagulation, and liver function tests, were normal. On admission, an enhanced computerized tomography (CT) scan of the upper abdomen demonstrated a 9.5 cm x 9.0 cm x 7.0 cm hypointense mass occupying the right liver (Figure 1), which was diagnosed as a primary right liver tumor. After completing relevant examinations, LARH was performed. We selected this case to demonstrate how the surgery was performed across a cohort of 82 patients.
Protocol
This study was approved by the Zhujiang Hospital of Southern Medical University Committee (Ethics Approval No: 2021-KY-081-01) on August 25, 2021. The present human surgery protocol was approved by and performed according to the ethical guidelines of the Zhujiang Hospital, Southern Medical University (Guangzhou, China). Moreover, informed consent was obtained from the patient for the release of his treatment-related information and data.
1. Inclusion and exclusion criteria
- Include patients with the following clinical evaluations.
- Preoperative CT image evaluation suggestive of hepatocellular carcinoma.
- Preoperative imaging examination indicating that the maximum diameter of liver tumors is >5 cm.
- All preoperative Child-Pugh grades as A or B9.
- Patients with residual volumes of >30% normal and >40% sclerotic livers.
- Patients with no serious vital organ lesions.
- Exclude patients with the following clinical evaluation.
- Preoperative CT images suggestive of tumor invasion of the IVC, left hepatic pedicle or left hepatic vein.
- Preoperative CT images indicating tumor invasion of the left liver and distant metastasis.
- Preoperative CT images suggestive of tumor rupture.
- Preoperative CT images showing the presence of tumor close to the IVC.
2. Preoperative preparation
- Prohibit the patient from eating and drinking before the surgery.
- Use tracheal intubation under general anesthesia10.
- Sterilize the skin with 0.5% iodine-based scrub.Place sterile towels on the inter-nipple connection, symphysis pubis, right midaxillary, and left midclavicular lines.
NOTE: Disinfect the surgical area with iodophor three times.
3. LARH surgical technique
- Place the patient in a supine position, with the head of the bed elevated, the feet of the bed lowered, and the right side of the patient's body tilted at 15°.
- Make five different curved incisions. Use a pneumoperitoneum needle to access the abdominal cavity by inserting it through the umbilicus incision. Connect the pneumoperitoneum needle to the pneumoperitoneum machine through the pneumoperitoneum connecting tube and inject CO2 into the abdominal cavity (Table of Materials). Set the pneumoperitoneum pressure to 13 mmHg on the pneumoperitoneum machine.
- Consider a curved incision on the umbilicus as the laparoscopic hole (A), two 1-cm curved incisions (B and E) as the main operating holes, and two 5-mm curved incisions (C and D) as the auxiliary operating holes (Figure 2).
- Place the trocar of 5, 10, 10, 5, and 5 mm at the umbilicus (Figure 2, point A), below the xiphoid process (point B), midclavicular line (point E), the anterior axillary line under the right costal margin (point C), and between operation incisions A and B (point D) (Figure 2).
- Perform abdominal exploration by penetrating the abdominal cavity. Locate the tumor and check for the presence of significant extrahepatic metastases through visual inspection.
- Dissect the recess6 between the root of the middle hepatic vein (MHV) and the right hepatic vein (RHV) with an ultrasonic knife and an aspirator (Figure 3).
- Separate the gallbladder triangle with an ultrasonic knife to expose the gallbladder duct and artery. Cut them off and remove the gallbladder.
- Ensure the assistant assists in lifting the liver, and the operator separates the right hepatic artery (RHA) and the right branch of the portal vein (RPV) with an ultrasonic knife and aspirator. Then, use a suture (4#) to ligate the RPV but do not transect it first. Use a home-o-lock to clamp the RHA.
- Trace the IVC bottom-up to look for the short hepatic veins (SHVs) wounds and ligate them (Figure 4A). Then clamp them using an ultrasonic knife to access the avascular area behind the liver (Figure 4B).
- Insert the Goldfinger dissector through the hepatic posterior space and exit through the hepatic vein recess.
- Fix the Goldfinger dissector with a urinary catheter (Table of Materials) and bypass the liver to establish a retro hepatic tunnel (Figure 5).
- Use a catheter to lift the liver and assist in exposing the liver resection plane during liver dissection.
- Transect the liver parenchyma with an ultrasonic knife along the hepatic ischemic line. Ensure no significant bleeding occurs until the tumor is removed along with the right liver.
- Open the inferior hepatoduodenal ligament with an ultrasonic knife and use the sterile bracelet to bypass the hepatoduodenal ligament as a pre-blocking band to perform the first hilar occlusion and reduce bleeding, if necessary.
- When possible, use an ultrasonic knife to transect the liver parenchyma along the MHV and during the process. Thicker pipes that are encountered can be cut off by suture ligation and home-o-lock clipping (Figure 6).
- Free the right anterior and posterior Glissons and subsequently transect them using an endoscopic stapling instrument and single-use loading unit (e.g., Endo-GIA).
- After completely cutting off the liver parenchyma, examine the presence of vessels from the bottom up and separate them one by one.
- Separate the right hepatic coronary and triangular ligaments using an ultrasonic knife with the help of an assistant who can help with proper exposure.
- Place the specimen in a specimen bag and make a transverse incision at the intersection of the right midclavicular line and the umbilical line (Line Red) to remove the specimen altogether (Figure 2).
NOTE: Note that the MHV is visible during the liver section and IVC below the liver after completing liver resection (Figure 7). - Place a laparoscopic drainage tube (Table of Materials) at the liver resection site and exit from the right lower abdomen after confirming no active bleeding in the abdominal cavity.
- Suture all the 5-mm and 10-mm trocar incisions layer by layer.
4. Postoperative care
- Closely monitor the patient's vital signs in the first postoperative 24 h through continuous real-time electrocardiography.
- Administer an antibiotic intravenously for 24 h postoperatively to prevent infection.
- Remove the catheter and drainage tube after 24 h and 48-72 h postoperatively, respectively.
- Perform an enhanced CT examination on the 3rd postoperative day.
NOTE: Enhanced CT showed changes after the right hepatocellular carcinoma was resected, with an indentation corresponding to the external drainage tube in the operative area (Figure 8).
Results
The relevant outcomes of LARH are shown in Table 1. The patient featured in the video recovered well after the surgery and was sent back to the ward. The operation lasted 180 min, with an intraoperative blood loss of approximately 150 mL that did not require a blood transfusion. The intraoperative urinary output was 800 mL. The time for establishing the retro hepatic tunnel and transecting the liver parenchyma are 15 min and 35 min respectively. The Pringle maneuver was performed twice. The patient recovered well without postoperative complications and was discharged on the 8th postoperative day. Preoperative CT showed a moderately poorly differentiated hepatocellular carcinoma measuring 9.5 cm x 9.0 cm x 7.0 cm (Figure 1). Postoperative CT revealed complete resection of the right liver tumor, confirming R0 resection, without any significant effusion in the right liver section (Figure 8). Postoperative pathological results confirmed hepatocellular carcinoma (Figure 9). The disease-free survival (DFS) of the patient is 17 months and overall survival (OS) is 32 months.
The above case was selected to demonstrate how the surgery was performed in 82 patients. From December 2015 to June 2022, 82 patients diagnosed with large right hepatocellular carcinoma (maximum tumor diameter ≥5 cm) were recruited for the study. Overall, 54 and 28 patients underwent LARH and LCRH, respectively. LARH was performed as described above and LCRH was performed as described previously3 (see Supplementary File 1).
The perioperative clinical data and survival outcomes of the two groups were compared. The characteristics of all 82 patients are summarized in Table 2.There was no significant difference between the LARH and LCRH groups with respect to clinical characteristics (p >0.05). The surgical results are summarized in Table 3. The intraoperative blood loss, duration of the technique, liver parenchyma transection time, and complication rates in the two groups can be determined from the results. The above perioperative observation indicators demonstrate the effectiveness and safety of LARH. The intraoperative blood loss among the patients in the LARH group was less than that of the patients in the LCRH group (200 vs. 300 mL, p < 0.05). The retro hepatic tunnel was successfully established in patients who underwent LARH without massive hemorrhage, and the catheter was used to complete the hanging maneuver. The median time for establishing the retrohepatic tunnel in patients in the LARH group was 15 min. The liver parenchyma transection time among patients in the LARH group was less (p = 0.011). Furthermore, the patients in the LARH group had shorter postoperative hospital stays (8.5 vs. 11 days, p <0.05). The complication rates (grades III and IV) according to the Clavien-Dindo classification11 in the LARH group were better than those in the LCRH group (9.3% vs. 32.1%, p = 0.009). The two groups of patients successfully completed the operation, and the survival rate was 100% right after the operation.
Four of the 82 patients did not turn up for their follow-up visits and 78 patients were included in the survival analysis for a follow-up of 8-69 months, with a median follow-up of 32 months. In the LARH group, the 1, 3, and 5-year disease free survival (DFS) rates were better than those in the LCRH group (88.5% vs. 76.9%, 65.5% vs. 42.0%, 48.9% vs. 29.4%, respectively; p = 0.043)(Figure 10). The 1, 3, and 5 year OS rates were similar in the LARH group (95.5% vs. 96.2%, 70.8% vs. 64.2%, 84.5% vs. 64.2%, respectively; p = 0.35) compared to those in the LCRH group (Figure 11).The prognostic factors for OS and DFS are shown in Table 4. Multivariate analysis indicated that LARH (hazard ratio [HR]=0.518, 95% CI 0.268-1.000, p=0.049), and no vascular tumor thrombus (hazard ratio [HR]=0.110, 95% CI 0.151-0.240, p<0.001), and blood loss < 250ml (hazard ratio [HR]=2.067, 95% CI 1.027-4.163, p<0.042) were associated with longer DFS. In addition, no vascular tumor thrombus (hazard ratio [HR]=0.229, 95% CI 0.106-0.493, p<0.001) was associated with longer OS.
Table 1: Relevant outcomes of LARH. a Clavien-Dindo classification grade III/IV Please click here to download this Table.
Table 2: Patient characteristics. HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein. Please click here to download this Table.
Table 3: Surgical results. a Clavien-Dindo classification grade III/IV Please click here to download this Table.
Table 4: Prognostic factor analysis for DFS and OS. HR: hazard ratio; OS: overall survival; DFS: disease-free survival; AFP: serum α-fetoprotein level. Data in parentheses are 95% confidence intervals. A Cox proportional hazard regression model for DFS and OS was used. Please click here to download this Table.
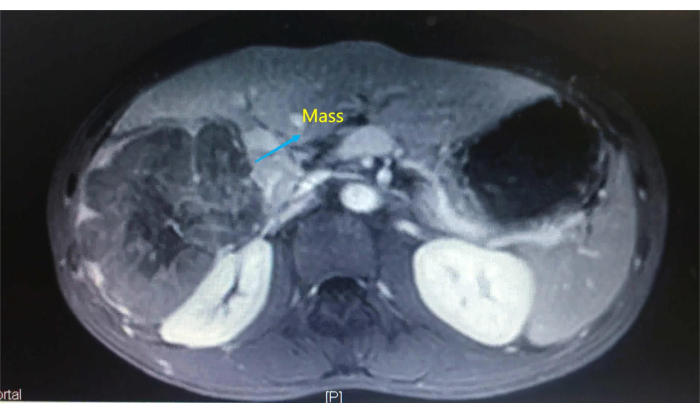
Figure 1: Enhanced computerized tomography confirmed a hypointense mass occupying the right liver. Please click here to view a larger version of this figure.
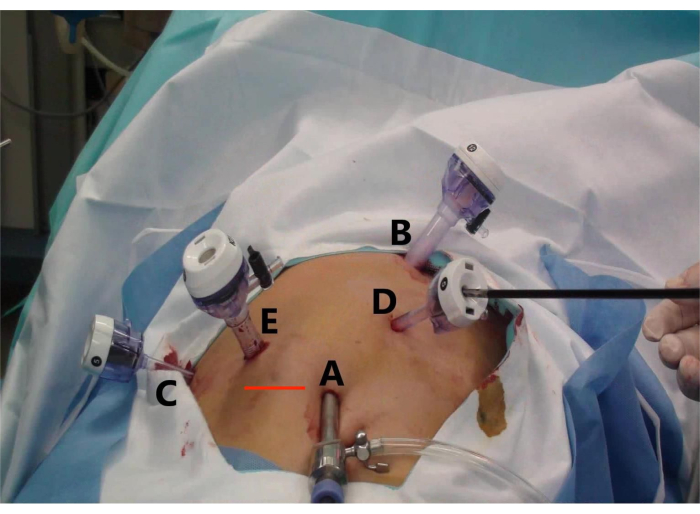
Figure 2: Trocar placement and specimen extraction incision for laparoscopic anterior right hepatectomy (LARH). The figure shows (A) the observation hole, (B) the main operation hole for the assistant, (C) the auxiliary operation hole for the operator, (D) the auxiliary operation hole for the assistant, and (E) the main operation hole for the operator, and (Line Red) the transverse incision to remove the specimen. Please click here to view a larger version of this figure.
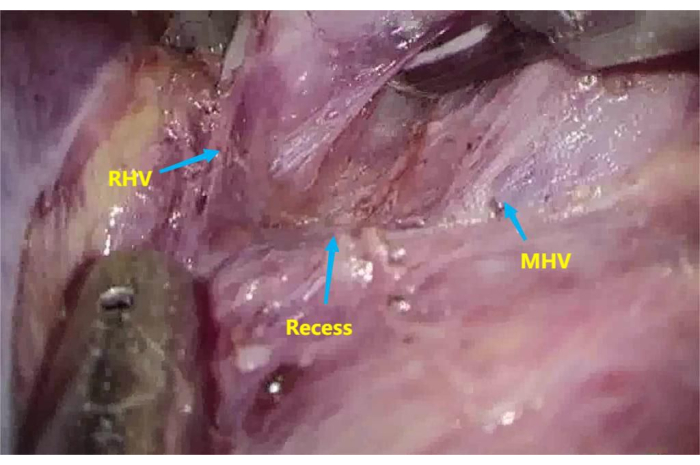
Figure 3: Recess between the root of the middle hepatic vein (MHV) and right hepatic vein (RHV). To dissect the recess between the root of MHV and RHV. Please click here to view a larger version of this figure.
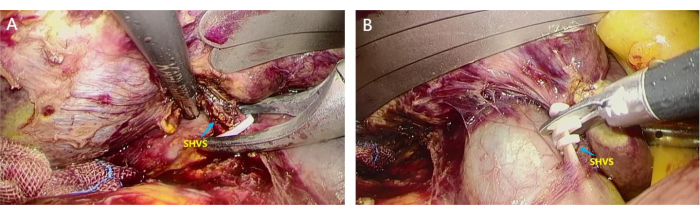
Figure 4: Dissection of short hepatic veins (SHVs). SHVs were separated and ligated to access the avascular area behind the liver. (A) The assistant lifts the liver, and the surgeon uses home-o-lock to clamp the thicker SHVSs; (B) SHVs were disconnected using a scissor to access the avascular area behind the liver. Please click here to view a larger version of this figure.
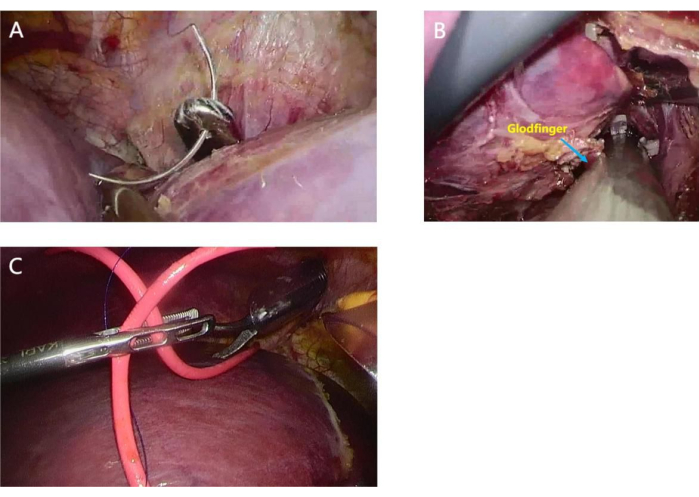
Figure 5: The use of Goldfinger dissector and urinary catheter to lift the liver. An 8-mm urinary catheter was fixed at the Goldfinger dissector, and the liver was bypassed to establish a retro hepatic tunnel. (A) The Goldfinger dissector bypassing behind the liver; (B) The insertion of the urinary catheter with a suture after the Goldfinger dissector emerged from the recess between the root of the middle hepatic vein (MHV) and right hepatic vein (RHV); (C)The urinary catheter lifting the liver after it passes through the retrohepatic tunnel. Please click here to view a larger version of this figure.
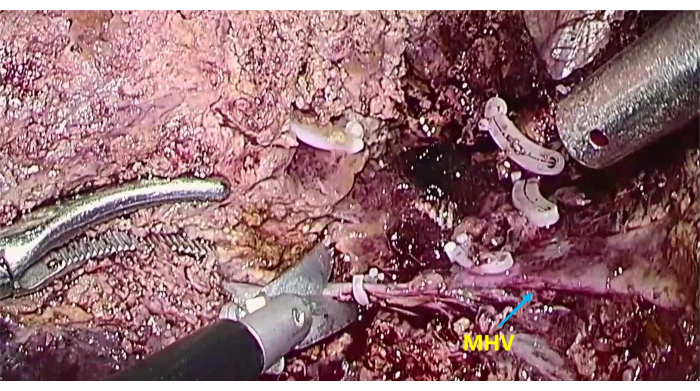
Figure 6: The exposure of middle hepatic vein (MHV) To transect the liver parenchyma along the MHV. Please click here to view a larger version of this figure.
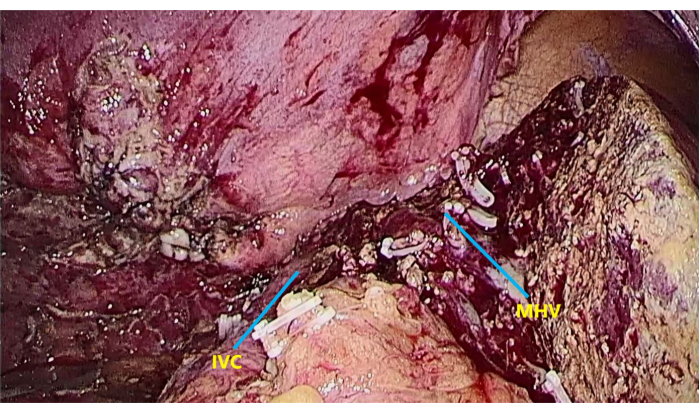
Figure 7: The exposure of middle hepatic vein (MHV) and inferior vena cava (IVC). To expose MHV and IVC after completing liver resection. Please click here to view a larger version of this figure.
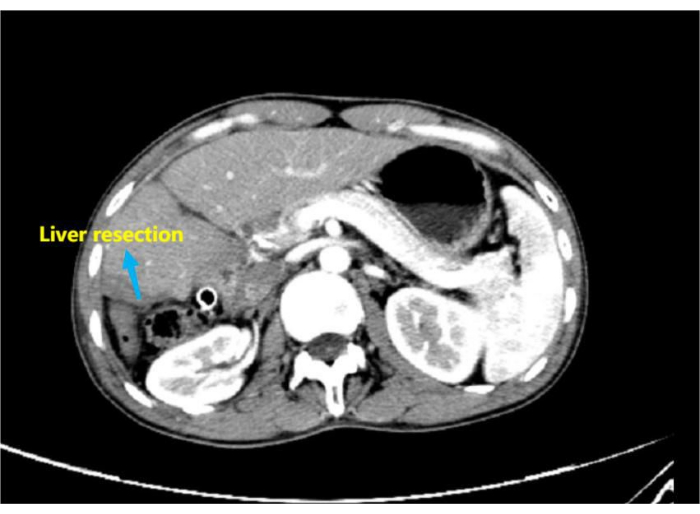
Figure 8: Postoperative enhanced computed tomography showing changes after resectioning the right hepatocellular carcinoma, suggesting a different resected right liver with indentation of an external drainage tube in the operative area compared with that observed during preoperative imaging. Please click here to view a larger version of this figure.
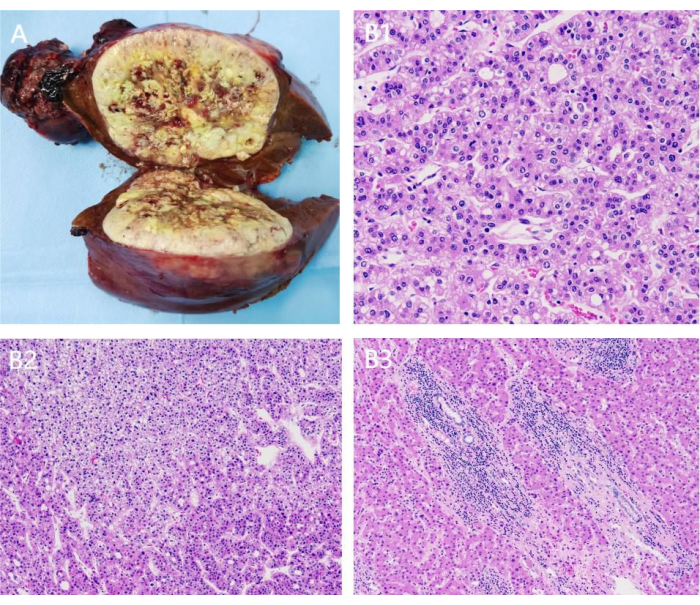
Figure 9: Postoperative pathological result. (A) The resected right liver tumor; (B1-3) The HE stains of the tumor confirm hepatocellular carcinoma. Please click here to view a larger version of this figure.
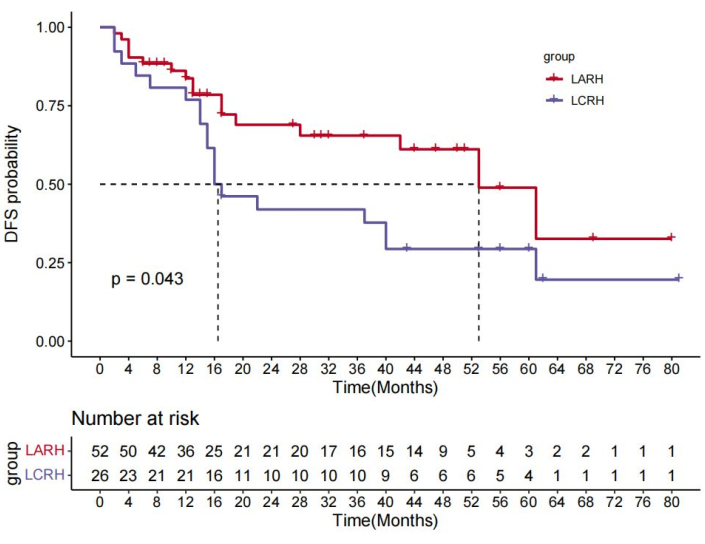
Figure 10: The disease-free survival (DFS) was better in the LARH group compared to those in the LCRH group, P = 0.043 (log rank test). Please click here to view a larger version of this figure.
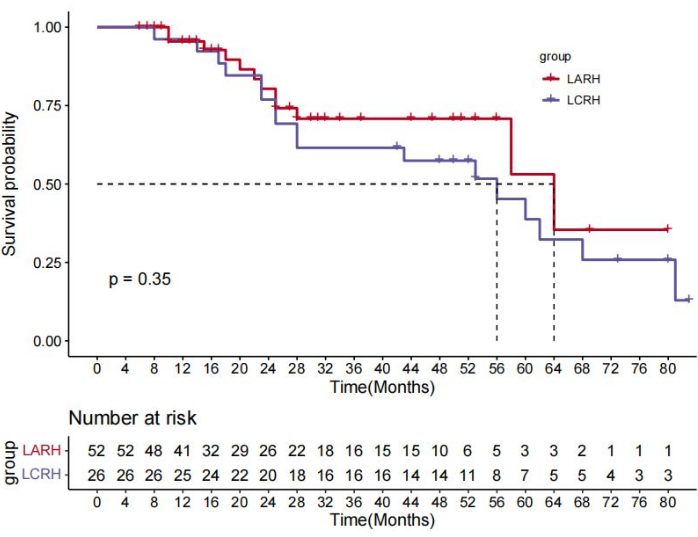
Figure 11: The overall survival (OS) was similar in the LARH group compared to those in the LCRH group, P = 0.35 (log rank test). Please click here to view a larger version of this figure.
Supplementary File 1: LCRH steps in brief. Please click here to download this File.
Discussion
Owing to the development of new surgical instruments and the advancement of anatomical theory12,13,14, AA-RH is currently widely applied in several medical centers worldwide. The prognosis of patients who undergo AA-RH has been shown to be superior to those who undergo the conventional procedure. However, research has indicated that tumor size (maximum tumor diameter ≥5 cm) may be an important clinical determinant of AA-RH success15. The AA-RH technique was originally developed through optimization of the CA-RH surgical approach, and its many advantages have since been proven. LARH and LCRH are procedures that combine the AA-RH and CA-RH techniques with laparoscopy, respectively. In this study, we found that LARH was superior to LCRH with regard to clinical effects and survival.
The hanging maneuver utilized in LARH facilitates easy exposure of the vascular structure, allowing optimal guiding of the resection path13,16. To implement this technique during our surgical procedures, we established a retro hepatic tunnel in the retro hepatic space, a region first described by Couinaud as a loose reticular space with few blood vessels between the liver and vein17. When establishing this tunnel, it is important to identify the gaps between the hepatic veins and to ligate the SHVs. Several materials can be used to lift the liver, including a rubber band, a cotton sling, or a homemade suspender18,19,20. In our center, we chose a urinary catheter because of its convenience. The hanging maneuver technique was employed to guide the cutting plane of the liver and increase the excision rate of a large laparoscopic hepatectomy. However, this technique cannot be used for tumors that adhere closely or invade the IVC, as these features lead to the failure of the established retro hepatic tunnel. In our case, patients in the LARH group showed significant reductions in blood loss, and the time required to transect the liver parenchyma was also less than in the LCRH group, indicating the safety and feasibility of this technique. Furthermore, the time required to establish the retro hepatic tunnel fell within an acceptable range, and the times of Pringle were similar between the two groups, confirming the effectiveness of this technique. Additionally, retro hepatic tunnel creation using the anterior approach was successful in all cases, as we excluded patients in whom the tumor adhered closely to the IVC.
The use of the anterior approach during hepatic parenchyma dissection prevents residual lesions while retaining as much of the normal liver as possible2,15. The most common postoperative complications in all patients were infection and liver insufficiency. Our results show that the conventional surgical approach was associated with excessive intraoperative blood loss and a possible increase in postoperative infection risk in the LCRH group. Additionally, the conventional approach increased the compression of the normal liver, resulting in increased liver insufficiency in the LCRH group compared to the LARH group. Finally, the length of hospital stay was shorter in the LARH group, indicating that this technique facilitates a faster postoperative recovery after an operation. Several studies have confirmed that the rates of major complications could be reduced by applying this technique in the place of the conventional approach21,22.
Perihepatic mobilization may cause iatrogenic tumor extrusion and rupture, facilitating the spread of cancer cells into the systemic circulation, and thereby significantly increasing the risk of tumor dissemination and recurrence22,23,24. Conversely, LARH is a non-contact and non-extrusion technique in which hepatic blood flow is controlled before separating the liver to avoid tumor spread and effectively reduce postoperative tumor recurrence rate24,25,26. Nevertheless, postoperative recurrence of hepatocellular carcinoma remains an important consideration for hepatobiliary surgeons. Further, the DFS rate of hepatocellular carcinoma after surgery is an important factor affecting patients' prognosis. As such, the evaluation of DFS and OS rates is essential when judging the effectiveness of a surgical procedure. Our analysis revealed similar OS rates between the LARH and LCRH groups; however, the LARH group had a superior DFS rate, which may have been caused by the small sample size in our study. Multivariate analysis of the Cox proportional regression risk model demonstrated that treatment with LARH, the absence of vascular tumor thrombus, and blood loss < 250ml were all associated with a longer DFS. Our findings confirm the superior prognosis of LARH vs LCRH, which is consistent with the results of most contemporary studies. Therefore, selecting LARH in appropriate cases is important to improve patient prognosis. However, tumor diameter and AFP level were not found to be risk factors for DFS, which may be related to the small sample size.
This study had some limitations, including the steep learning curve, long study period, and selection bias associated with retrospective studies. The steep learning curve may be attributed to the author's understanding of the advantages of the anterior approach, making it easier to select this approach later in the study. In addition, the conventional approach was selected predominantly in the early stage of the study, whereas the anterior approach was more commonly selected in the latter half. These factors may have affected the choice of surgical methods and comparison of clinical effects. Additionally, the sample size was small. Future studies with a larger sample size are required to fully reveal the significance and effectiveness of LARH.
Based on our results, we concluded that LARH can effectively reduce blood loss, accelerate liver transaction, and reduce tumor recurrence compared with LCRH. LARH involves less contact and extrusion, which agrees with the "tumor-free principle". Therefore, we propose that LARH could be a useful treatment strategy for large right hepatocellular carcinoma.
Disclosures
The authors declare that they have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82072627).
Materials
| Name | Company | Catalog Number | Comments |
| Pneumoperitoneum needle | Unimicro Medical Systems Co.,Ltd | 150mm | |
| Disposable single-cavity rubber catheter | Yangzhou Huayue Technology Development Co, Ltd | 3.5mm (10Fr) | |
| Disposable spiral negative pressure drainage pipeline | Jiangsu Aiyuan Medical Technology Corp | 424280 | |
| Disposable trocar | Kangji Medical | 10010, 10012 | |
| Electrocardiographic monitor | Philips Goldway (SHENZHEN) Industrial, Inc | UT4020B | |
| Endoscopic Stapling Instrument & Single Use Loading Unit (Endo-GIA) | Covidien | 1650 | |
| Laparoscopic system | Olympus | WM-NP2 L-RECORDOR-01 | |
| Non-absorbable polymer ligation clips (Home-o-lok) | Teleflex Medical | 545330 | |
| Ultrasound knife | Johnson | GEN11 | |
| Video system | Lenovo | GK309 |
References
- She, W. H., et al. Anterior approach to major resection for colorectal liver metastasis. J Gastrointest Surg. 11, 1928-1938 (2018).
- Beppu, T., et al. Anterior approach for right hepatectomy with hanging maneuver for hepatocellular carcinoma: a multi-institutional propensity score-matching study. J Hepatobiliary Pancreat Sci. 3, 127-136 (2017).
- Tang, J. X., Li, J. J., Weng, R. H., Liang, Z. M., Jiang, N. Anterior vs conventional approach right hepatic resection for large hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastroenterol. 44, 7917-7929 (2017).
- Peng, Y., et al. Is the anterior approach suitable for laparoscopic right hemihepatectomy in patients with large hcc (5-10 cm)? A propensity score analysis. Surg Endosc. 8, 6024-6034 (2022).
- Lai, E. C., Fan, S. T., Lo, C. M., Chu, K. M., Liu, C. L. Anterior approach for difficult major right hepatectomy. World J Surg. 3, 314-317 (1996).
- Belghiti, J., Guevara, O. A., Noun, R., Saldinger, P. F., Kianmanesh, R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 1, 109-111 (2001).
- Troisi, R. I., Montalti, R. Modified hanging maneuver using the Goldfinger dissector in laparoscopic right and left hepatectomy. Dig Surg. 6, 463-467 (2012).
- Cai, L. X., Wei, F. Q., Yu, Y. C., Cai, X. J. Can retrohepatic tunnel be quickly and easily established for laparoscopic liver hanging maneuver by Goldfinger dissector in laparoscopic right hepatectomy. J Zhejiang Univ Sci B. 9, 712-721 (2016).
- . EASL Clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 69, 182-236 (2018).
- Costi, R., et al. Partial splenectomy: who, when and how, a systematic review of the 2130 published cases. J Pediatr Surg. 54, 1527-1538 (2019).
- Dindo, D., Demartines, N., Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2, 205-213 (2004).
- Yoh, T., et al. Laparoscopic right hepatectomy using the caudal approach is superior to open right hepatectomy with anterior approach and liver hanging maneuver: A comparison of short-term outcomes. Surg Endosc. 2, 636-645 (2020).
- Hasegawa, Y., et al. Anterior approach for pure laparoscopic donor right hepatectomy. Surg Endosc. 10, 4677-4678 (2020).
- Chu, H., et al. Laparoscopic liver hanging maneuver through the retrohepatic tunnel on the right side of the inferior vena cava combined with a simple vascular occlusion technique for laparoscopic right hemihepatectomy. Surg Endosc. 6, 2932-2938 (2018).
- Chen, H. W., et al. Laparoscopic right hepatectomy via an anterior approach for hepatocellular carcinoma. JSLS. 1, e2017.00084 (2018).
- Araki, K., et al. Left lobe mobilization strategy of right-sided major hepatectomy for treatment of a tumor causing severe inferior vena cava compression: a novel strategy using the modified liver-hanging maneuver. Ann Surg Oncol. 5, 1150-1151 (2018).
- Couinaud, C. Exposure of the left hepatic duct through the hilum or in the umbilical of the liver: Anatomic limitations. Surgery. 1, 21-27 (1989).
- Nanashima, A., Nagayasu, T. Development and clinical usefulness of the liver hanging maneuver in various anatomical hepatectomy procedures. Surg Today. 4, 398-404 (2016).
- Ariizumi, S. I., Nanashima, A., Yamamoto, M. Anterior approach in right hepatectomy. J Hepatobiliary Pancreat Sci. 7, 351-352 (2018).
- Sugimachi, K., Iguchi, T., Nakanoko, T., Mano, Y. Inferior right hepatic vein-preserving hepatectomy for large liver tumors: anterior approach using the two-stage modified hanging maneuver (with video). J Gastrointest Surg. 6, 1444-1447 (2020).
- Llado, L., et al. The anterior hanging-approach improves postoperative course after right hepatectomy in patients with colorectal liver metastases. results of a prospective study with propensity-score matching comparison. Eur J Surg Oncol. 2, 176-183 (2016).
- Zheng, J., et al. Outcomes of anterior approach major hepatectomy with diaphragmatic resection for single huge right lobe HCC with diaphragmatic invasion. Medicine (Baltimore). 36, e12194 (2018).
- Zhang, Y., et al. Long-term survival after anterior approach right hepatectomy combined with inferior vena cava thrombectomy using trans-diaphragmatic intrapericardial inferior vena cava occlusion: a case report and review of the literature. BMC Surg. 1, 122 (2019).
- Ratti, F., Cipriani, F., Catena, M., Paganelli, M., Aldrighetti, L. Approach to hepatocaval confluence during laparoscopic right hepatectomy: three variations on a theme. Surg Endosc. 2, 949 (2017).
- Jackson, N. R., et al. The safety and efficacy of approaches to liver resection: a meta-analysis. JSLS. 1, e2014-e2186 (2015).
- Chen, H. W., et al. Anterior approach for right hepatectomy using the 5-steps stapling technique: a preliminary study. Int J Surg. 32, 19-23 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved