Method Article
Bioassay-Guided Identification of Natural Products for Biocontrol by Thin Layer Chromatography-Direct Bioautography
In This Article
Summary
We describe the use of thin-layer chromatography direct bioautography assay and liquid chromatography-mass spectrometry to identify microbial natural products that display antagonism against fungal pathogens using the pathogen Sclerotinia sclerotiorum and biopesticidal Bacillus isolates as a model system.
Abstract
Thin layer chromatography-direct bioautography (TLC-DB) is a well-established bioassay used to separate and identify natural products (NPs) that are antagonistic against a target pathogen. It is a rapid, inexpensive, and simple option for the bioassay-guided isolation and identification of NPs that hinges on separation by TLC coupled with the direct application of a target pathogen to examine bioactivity. It is typically used for the analysis of bioactive plant extracts, detecting inhibitory activity against bacteria, fungi, and enzymes. That being said, it has great potential in bacterial NP discovery, particularly for evaluating bacterial NPs against pertinent agricultural pathogens, which is valuable for discovering and developing novel biopesticides for the agriculture industry. Furthermore, it is a tunable protocol that could be applied to other target pathogens or sources of NPs in research programs concerning the discovery and identification of bioactive compounds. Herein, we describe a model system for discovering and identifying biopesticide NPs using TLC-DB with Bacillus spp. and the agricultural pathogen Sclerotinia sclerotiorum.
Introduction
Fungal agricultural pathogens cause significant losses in crop quality and yields worldwide, contributing to the economic and supply challenges of a stable global food production system1,2. Pathogen damage can be prevented by breeding cultivars resistant to infection and using integrated crop management systems, including crop rotations and land management practices to suppress pathogen proliferation3,4. Though these methods reduce damage to crops, chemical pesticides are generally used in tandem to actively kill fungal reproductive structures in the field and further prevent damage and yield reductions. Although effective, chemical pesticide usage has many drawbacks, including damage to surrounding ecosystems, a decline in soil fertility, associated human health risks, and the development of pathogen resistance, the latter causing higher doses of pesticides to be required each year5,6,7.
Microbial-based pest and pathogen management products have long been considered potential alternatives or complementary to synthetic pesticides. Since the early 1900s, Bacillus thuringiensis has been widely used to control agricultural pests and pathogens as a seed treatment, as a foliar spray, and in direct soil treatment8. Such products have been named biopesticides and are characterized as naturally occurring microorganisms or biochemicals that can kill, suppress, or reduce the vigor of a target pest or pathogen. Biopesticides can control the growth of a pathogen through various mechanisms but most commonly do so through the secretion of secondary metabolites9. Secondary metabolites, often referred to as natural products, are not involved in primary metabolism but are produced as an evolutionary advantage to outcompete other microorganisms10.
Biopesticides offer many advantages over their synthetic counterparts. They pose a low toxicity risk to the environment, fauna, and humans compared to synthetic pest management products9,10. Since most biopesticides have existed naturally in the environment for millennia, biodegradation pathways for microbial metabolites exist in the environment, limiting the possibility of soil or ecosystem contamination and reducing residence times that contribute to synthetic pesticides being so environmentally destructive11. Additionally, many biopesticides used for mitigating pathogen infection also exhibit plant growth-promoting properties, which can increase nutrient bioavailability and induce plant systemic resistance12.
Most commonly, biopesticides are applied in the form of microbial inoculum, and NPs are secreted by live microorganisms in situ12,13. In such a case, identifying the source of the activity of a biopesticide is of great value. Doing so provides insight into the mechanism of action of the biopesticide, aids with building a case for the protection of a microorganism with a patent, and can have a significant scientific impact if their structures are novel. Most importantly, however, identification of the bioactive source informs formulation possibilities for a downstream biopesticide product. If the NP itself is active, one can use the microorganism as a biomolecule factory for large-scale biopesticide production. Additionally, many NPs that have been explored for biocontrol also have potential applications in human medicine, making them even more economically valuable8.
Thin layer chromatography-direct bioautography (TLC-DB) assays are an inexpensive and straightforward method for the bioactivity-guided isolation and identification of biopesticidal metabolites. Although the technique is commonly used for separating bioactivity testing of phytochemicals from crude plant extracts, it also has great potential for the analysis of microbial extracts14. TLC provides fast and inexpensive separation of NPs in a crude microbial extract, and after coating with a media pathogen suspension, zones that contain active metabolites are easily visualized. Those zones can be scraped from the plate and extracted for chemical analysis by ultra-high-performance liquid chromatography coupled with mass spectrometry (UPLC-MS) to identify known metabolites. Metabolites that do not match previously reported compounds can be isolated in larger quantities via liquid chromatography to undergo structure elucidation studies using techniques such as nuclear magnetic resonance spectroscopy and X-ray crystallography15.
This article describes a model system for discovering and identifying biopesticide NPs using TLC-DB with Bacillus spp. and the agricultural pathogen Sclerotinia sclerotiorum. Figure 1 provides a schematic overview of the TLC-DB procedure.
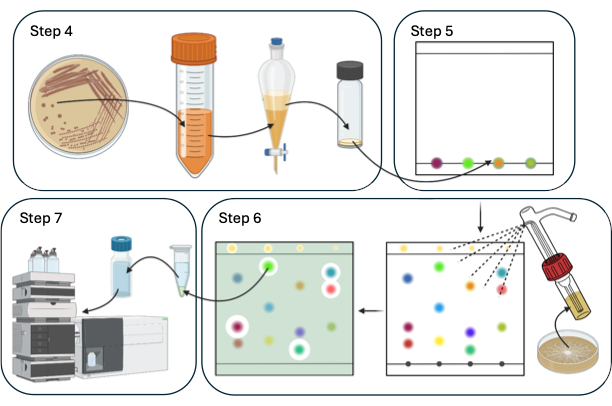
Figure 1: Schematic overview of steps 4-7 of the procedure of TLC-DB. Please click here to view a larger version of this figure.
Protocol
The details of the reagents and the equipment used in this study are listed in the Table of Materials.
1. Selecting the microbial biocontrol candidates
- Using competition plate assays, select microbial isolates that display antagonism against the pathogen of interest.
NOTE: Nine Bacillus isolates that exhibited antagonism against S. sclerotiorum were selected, including Bacillus atrophaeus, mojavensis and subtilis species, to demonstrate this protocol.
2. Media preparation
- Prepare potato dextrose agar (PDA) by dissolving 39 g of medium per liter of water.
- Prepare Pseudomonas F agar by adding 45 g of media per liter of water.
- Prepare potato dextrose broth (PDB) with 1% agar by adding 24 g of medium, and 0.24 g agar per liter of water.
- Prepare yeast-glucose-manganese (YGM) broth. Create a buffer solution consisting of 2.5 g of KH2PO4 and 2.5 g K2HPO4 in 100 mL of water, and a salt solution consisting of 0.58 g of MnSO4.H20, 0.5 g of NaCl and 0.05 g of FeSO4.7H20 in 100 mL of water.
- Next, add 1 g of yeast extract, 1.25 g of dextrose, 400 mL of water, 5 mL of the buffer solution, 5 mL of the salt solution and 90 mL of water to ensure that the metal salts do not precipitate in solution.
- Autoclave-sterilize the media, pour the agar into 100 x 15 mm Petri plates, and let all cool to room temperature before use.
3. Pathogen preparation
- Culture five plates of Sclerotinia sclerotiorum on PDA (prepared in step 2.1) and incubate at 25 °C for 3 days.
4. Natural product extract preparation
- Culture each bacterial isolate on Pseudomonas F Agar (prepared in step 2.2) and grow until individual colonies are observed.
- Subculture single bacterial colonies into 25 mL of YGM media in centrifuge or culture tubes and incubate with shaking for 3 days.
- Transfer 5 mL of aliquots of each extract into 1 L of YGM and incubate in the same conditions for another 3 days.
- Centrifuge each culture at 3000 x g for 15 min at 25 °C to pellet the cells.
- Decant and wash the supernatant three times with ethyl acetate to extract metabolites from the culture medium.
- Combine and dry the ethyl acetate layer of each using rotary evaporation.
- Re-dissolve the extract in minimal methanol, transfer it to a small scintillation vial, and dry it again by rotary evaporation.
NOTE: If the extract dries to an oily or resinous material, lyophilize the material to obtain a dry powder that can be accurately weighed.
5. TLC plate preparation
- Prepare the 20 cm x 20 cm TLC plate by drawing a line 2 cm from the bottom and 2 cm from the top of the plate. On the bottom line, place nine dots 2 cm apart. Above the top line, place four dots 4 cm apart.
- Dissolve 5 mg of each extract in 15 µL of methanol and apply 5 µL of each extract on the nine dots marked on the bottom line using a pipette. Let each extract spot dry on the TLC plate and apply another 5 µL aliquot to the same spot. Repeat until all of the material is loaded onto the TLC plate.
- Develop the TLC plate in a developing tank using a 1:2 solution of dichloromethane and methanol until the solvent reaches the line marked 2 cm from the top of the plate (approximately 45 min).
- Once developed, remove the TLC plate and apply a series of positive controls on the four dots marked above the solvent line. Four concentrations of the same control are used. For S. scl., 0.5 µg/mL, 5 µg/mL, 50 µg/mL, and 500 µg/mL of Hygromycin B are positive controls.
- Before all solvent evaporates from the plate, place it in an ethanol-sterilized TLC plate box.
6. Direct bioautography assay
- Autoclave the materials to be used in the assay (glass components of the chromatography sprayer, metal spatula, four sheets of paper towel, cellulose or filter paper, water, and media) in a large beaker covered with tinfoil.
NOTE: Paper materials (paper towel, cellulose paper) should be wrapped in tin foil to avoid becoming wet in the autoclave. - Connect an air source, pressure gauge, and chromatography sprayer using ethanol-sterilized PTFE tubing. Connect a HEPA filter between the chromatography sprayer and the pressure gauge.
- Collect the mycelial mat of the five pathogen plates using the sterile metal spatula into a 50 mL centrifuge tube using a sterile spatula.
NOTE: Use a small volume of liquid broth to help dislodge the mycelia if needed, and transfer with a sterile pipette. - Add 25 mL of PDB amended with agar and sterile glass beads to the 50 mL centrifuge tube and vortex for 5 min to break up the mycelial mat.
- Transfer the mycelial suspension to a chromatography sprayer using a sterile syringe with a needle tip to ensure large pieces of mycelia will not clog the chromatography sprayer.
- Place the previously developed TLC plates on sterile paper towels in a laminar flow hood to reduce hand contact with the plate while applying the media suspension.
- Increase the air pressure to approximately 4 bars and apply three coats of the suspension to the TLC plate, allowing the plate to dry completely between applications.
NOTE: Three layers were found to be the optimal amount. Less than this, and the pathogen does not have enough media to grow on. More than this, the media is too thick, which may not allow for pathogen contact with active metabolites. - Add 500 mL of sterile water into a sterilized plastic plate box and place sterilized folded cellulose sheets or filter paper on each side to hold moisture.
- Place the completed assay plate onto four empty Petri dishes in the box.
- Incubate the assay for 3 days to 1 week or until an even layer of mycelia grows evenly across the TLC plate except for around the positive controls and zone of inhibition (ZOIs).
7. Liquid chromatography mass spectrometry analysis
- Image the completed assay using photography.
- Calculate the retention factor for each zone of inhibition by dividing the distance from the bottom line to the ZOI by the distance from the bottom line to the top line.
- Scrape the silica off the inhibition zones and place it into microcentrifuge tubes.
- Add 500 µL of methanol to each tube and vortex to extract the metabolites from the silica.
- Centrifuge at 8,500 x g at 20 °C for 10 min, and transfer the supernatant to a small vial.
- Evaporate to dryness by rotary evaporation or under a stream of nitrogen.
- Re-suspend the extract in 50 µL of methanol in an LC vial.
- Analyze the metabolites in the zone of inhibition by LC-MS and compare them to the metabolites in the crude extract to determine which metabolites in the crude extract are responsible for the inhibition of the pathogen16.
- Using the genus and species of the source microorganism and the masses identified in the ZOI, search the literature and relevant databases such as Antibase and the Dictionary of Natural Products to identify the metabolites.
Results
Upon the separation of microbial extracts by TLC, metabolites should be dispersed along the TLC plate vertically. Under visible light, it can be difficult to view metabolites that do not absorb in the visible light range. Thus, imaging under UV light can help view the separation of metabolites, as seen in Figure 2A,B. After the incubation period, the pathogen should appear to grow evenly across the entire plate except over the positive controls and the inhibition zones where active metabolites reside, pictured in Figure 2C.
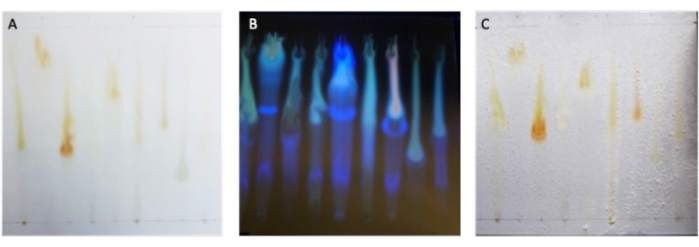
Figure 2: Separation of microbial extracts by TLC. (A) Developed TLC plate containing nine Bacillus extracts imaged under visible light and (B) under 320 nm UV light prior to the application of fungal inoculum. (C) Completed bioautography assay with pathogen growth observed across the plate except for where growth is inhibited around the positive controls, and ZOI of each extract. Please click here to view a larger version of this figure.
Metabolites extracted from the zones of inhibition are analyzed by LC-MS and compared to the crude extract to determine the NPs responsible for the antagonism against the pathogen. Matching metabolites should have the same retention time and molecular ion species to be considered a match. Once the active metabolites are identified, the bacterial culture can be grown in bulk to isolate active metabolites of interest for structural chemistry or biological study.
Discussion
TLC-DB is a valuable and well-established NP research tool and a simple and inexpensive alternative to microplate bioassay-guided isolation methods17. It requires minimal time and material resources compared to microplate assays, which require metabolite separation using liquid chromatography techniques. It is a highly versatile assay that can be used to detect antibacterial, antifungal, anti-parasitic, and antioxidant NPs in addition to enzyme inhibitors18,19,20,21,22,23. Though most commonly used for detecting and identifying bioactive phytochemicals, the same method can be applied for bacterial NPs as explored in this protocol18. Additionally, the protocol can be optimized for use with a variety of NP sources and target pathogens to aid in discovering and assessing novel bioactive NPs.
The media used for bacterial growth and solvent used for extraction can greatly impact natural product discovery results. The media used in this protocol is optimized for bacteria that produce cyclic lipopeptides, including Pseudomonas and Bacillus spp. but other media and nutrient sources should be considered if exploring other genera. One may choose to complete TLC-DB using the same microorganism grown in a range of media to evaluate the full breadth of metabolite diversity from one isolate or use identical growth conditions for a wider range of isolates. The choice of extraction solvent also impacts the natural products detected. It is generally understood that most bioactive NPs have low to moderate polarity, making ethyl acetate a suitable choice due to its low boiling point, which makes it easy to remove. However, if one also wishes to examine the polar and non-polar fractions, multiple extractions with other solvents can be carried out. Alternatively, the cell-free extract can be lyophilized and used in the assay to see all metabolites released into the media. However, more material will often need to be loaded onto the TLC plate to account for the dried media components in the lyophilized material. Similarly, if this method is used with a different pathogen, such as inoculum, the media used and incubation conditions must be optimized to obtain the 5 agar plates of mycelium used for the TLC-DB assay.
TLC-DB is advantageous compared to contact and immersion bioautography as it uses the thinnest layer of agar and inoculum, minimizing the reliance on the diffusion of metabolites into the agar layer, which can allow smaller amounts of natural products to induce pathogen inhibition17. Previously published findings using TLC bioautographic methods have used a spore suspension of the pathogen to complete the assay17. Although this does allow for precise control over the suspension concentration, it can be exceedingly difficult and time-consuming to induce the sporulation of certain fungi24. This modification greatly simplifies the assay and allows for the completion of the assay using fungal pathogens that are difficult to sporulate and may have previously been avoided for this method.
The mass of bacterial extract used in the assay can impact results. If too little extract is applied to the TLC plate, it is possible that the minimum inhibitory concentration of an active compound will not be surpassed, and bioactivity will not be detected. As a result, in some cases, and as seen in Figure 1 and Figure 2, it is worthwhile to overload the TLC plate, compromising separation for the ability to easily detect activity. Similarly, the pathogen load sprayed onto the plate must not be too low, as there will not be enough media applied to support pathogen growth. This method can be easily tuned to accommodate a variety of bacterial extracts and pathogens, and the quantities of microbial extract and inoculum outlined in the protocol have ensured bioactivity can be detected for multiple pathogens and microbial extracts. If no zones of inhibition are observed upon the completion of the assay, it may indicate one of the following. First, active metabolites may not be present in the extract applied due to incompatible media being used for bacterial growth or due to the activity of the bacteria not being a result of the NP production. A disc diffusion assay with the extract can be completed to confirm or deny the existence of active NPs in the extract. If the disk diffusion assay shows no pathogen suppression, other media can be tested to determine if other conditions produce bioactive NPs. If the disk diffusion assay does indicate that the extract suppresses the pathogen, then a larger mass of the bacterial extract may need to be applied to the TLC plate, in which case another assay can be attempted.
Comparing metabolites in the ZOI to the crude extract is essential for identifying active NPs. In the assay, metabolites from the crude extract can be metabolized or modified by the pathogen, which can be observed via LC-MS. Thus, only metabolites that occur in both the crude extract and the ZOI can be considered as NPs produced by the bacteria under study. If one cannot correlate the metabolites extracted from the ZOI to metabolites in the crude extract, a TLC plate can be prepared, as described in step 5. Without completing the bioautography assay, extract the metabolites from the TLC plate at the same retention time observed in the completed assay. This should allow for an easier correlation between the metabolites in the ZOIs and crude extract, causing pathogen suppression.
One drawback of TLC-DB is that the resolution of TLC is considerably less than that achieved when using traditional microwell screening techniques, which require liquid chromatography for separation. Thus, it is common for multiple metabolites to exist in the zone of inhibition where some metabolites may not be contributing to the bioactivity. This issue can be further caused by the practice of overloading the TLC plate to observe bioactivity more clearly. Recent work has been published using high-performance TLC (HP-TLC), which greatly improves resolution and can allow for the automation of TLC development that is otherwise impossible when using conventional TLC14,21,22,23. Also, plates can be developed in a second dimension (2D-TLC) to further separate metabolites with similar retention times. That being said, one should evaluate whether the increased time and material cost is a worthwhile compromise for increased resolution obtained from HP- and 2D-TLC25.
Disclosures
Dr. Susan Boyetchko passed away prior to the submission of this work (Feb 8, 2023). All other authors have declared to have no conflicts of interest.
Acknowledgements
We gratefully acknowledge Agriculture and Agri-Food Canada for the funding under which this research was made possible (projects J-001843 and J-002021). We thank Brett van Heyningen for filming the video content for this protocol. We would also like to thank former graduate students (Jennifer Vacon and Mark Nabuurs) for their insights into the methods described in this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 0.5-5 mL single channel Pipette | VWR | CA11020-004 | |
| 10 mL Thin Layer Chromatography Sprayer | VWR | KT422530-0010 | |
| 100 x 15 mm Petri plates | VWR | 89038-970 | |
| 100-1000 µL pipette tips | VWR | 76322-164 | |
| 100-1000 µL single channel pipette | VWR | 76169-240 | |
| 15 mL sterile centrifuge tubes | VWR | CA21008-918 | |
| 1 L glass bottle | Millipore Sigma | CLS13951L | Must be autoclave safe |
| 1 mL sterile syringe with needle | Thomas Scientific | 8935L75 | Detachable needle is recommended |
| 2 mL Microcentrifuge tube | VWR | 87003-298 | |
| 50 mL sterile centrifuge tubes | VWR | CA21008-940 | |
| 5 mL pipette tips | VWR | CA11020-008 | |
| 7 mL scintilation vials | VWR | 76538-962 | |
| 95% ethanol | Thermo Fisher Scientific | A412-500 | |
| Autoclave | Cole-Parmer | UZ-01850-34 | 8 L, 115 VAC |
| Bacteriological agar | VWR | 97064-336 | |
| bin | Thomas Scientific | 1216H91 | |
| D-Glucose | VWR | BDH9230-500G | |
| Dichloromethane ≥99.8% ACS | VWR | BDH1113-4LG | |
| Ethyl Acetate ≥99.8% ACS | VWR | BDH1123-4LG | |
| Filter paper | VWR | CA28297-846 | |
| Grinding Beads | VWR | 12621-148 | |
| Hygromycin B | VWR | CA80501-074 | |
| Iron Sulfate Heptahydrate | VWR | 97061-542 | |
| Laminar flow hood | CleanTech | 1000-6-A | |
| LC-MS | Waters | LITR10064178 | UPLC/MS/MS TQD system |
| Lyophilizer | Labconco | 700201000 | Temperature collector -50 °C |
| Manganese Sulfate Hydrate | VWR | CAAA10807-14 | |
| Methanol ≥99.8% ACS | VWR | BDH2018-5GLP | |
| Paper towel | VWR | 89402-824 | |
| Potato Dextrose Agar | VWR | CA90000-758 | |
| Potato Dextrose Broth | VWR | CA90003-494 | |
| Potsasium Phosphate Dibasic | VWR | 470302-246 | |
| Potsasium Phosphate Monobasic | VWR | 470302-252 | |
| Pressure Gauge 6mm Union Straight 0-10 bar (0-145 psi) | Tameson | F25U6 | |
| Pseudomonas F Agar | VWR | 90003-352 | Also known as Flo Agar |
| PTFE Tubing | Sigma Aldrich | 58697-U | 1/16 inch inner diameter |
| Sodium Chloride | VWR | BDH9286-500G | |
| Spatula | VWR | 82027-490 | |
| Sterile Inoculation loops with needle | VWR | 76534-512 | |
| Tinfoil | Thomas Scientific | 1086F24 | Can be purchased from supermarket |
| TLC Silica Gel 60 RP-18 F254S 25 Glass Plates 20 X 20 cm | Thomas Scientific | 1205Q12 | |
| Vacuum Pump | Labconco | 1472100 | 98 L/min |
| Vortex | VWR | 76549-928 | Must accomadate 15 mL and 50 mL centrifuge tubes |
| Whatman in-line HEPA-VENT | Millipore Sigma | WHA67235000 | 10 filters, 1/4 to 3/8 inch inlet/outlet |
| VWR | 97063-370 |
References
- Fisher, M. C., et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 484 (7393), 186-194 (2012).
- Savary, S., et al. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 3 (3), 430-439 (2019).
- Lin, B. B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. BioSci. 61 (3), 183-193 (2011).
- Piquerez, S. J., Harvey, S. E., Beynon, J. L., Ntoukakis, V. Improving crop disease resistance: Lessons from research on arabidopsis and tomato. Front Plant Sci. 5, 671(2014).
- Damalas, C., Koutroubas, S. Current status and recent developments in biopesticide use. Agriculture. 8 (1), 13(2018).
- Syed Ab Rahman, S. F., Singh, E., Pieterse, C. M. J., Schenk, P. M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 267, 102-111 (2018).
- Tudi, M., et al. Exposure routes and health risks associated with pesticide application. Toxics. 10 (6), 335(2022).
- Santos, E. N., et al. Bacillus thuringiensis: From biopesticides to anticancer agents. Biochimie. 192, 83-90 (2022).
- Kumar, J., Ramlal, A., Mallick, D., Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants. 10 (6), 1185(2021).
- Marrone, P. Pesticidal natural products - status and future potential. Pest Manag Sci. 75 (9), 2325-2340 (2019).
- Ayilara, M. S., et al. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 14, 1040901(2023).
- Abdelaziz, A. M., et al. Biocontrol of soil borne diseases by plant growth promoting rhizobacteria. Trop. Plant Pathol. 48 (2), 105-127 (2023).
- Zhao, Z., Liu, D., Ruan, L., Wang, T., Liang, Z. Antifungal mechanism of Bacillus amyloliquefaciens SC-B15 and its application in cereal mildewproof and grape preservation. Food Biosci. 56, 103287(2023).
- Jamshidi-Aidji, M., Dimkic, I., Ristivojevic, P., Stankovic, S., Morlock, G. Effect-directed screening of bacillus lipopeptide extracts via hyphenated high-performance thin-layer chromatography. J Chromatogr A. 1605, 460366(2019).
- Prichystal, J., Schug, K. A., Lemr, K., Novak, J., Havlicek, V. Structural analysis of natural products. Anal Chem. 88 (21), 10338-10346 (2016).
- De Souza, C. G., et al. Simultaneous quantification of lipopeptide isoforms by UPLC-MS in the fermentation broth from Bacillus subtilis CNPMS22. Anal Bioanal Chem. 410 (26), 6827-6836 (2018).
- Dewanjee, S., Gangopadhyay, M., Bhattacharya, N., Khanra, R., Dua, T. Bioautography and its scope in the field of natural product chemistry. J Pharm Anal. 5 (2), 75-84 (2015).
- Choma, I., Jesionek, W. TLC-direct bioautography as a high throughput method for detection of antimicrobials in plants. Chromatography. 2 (2), 225-238 (2015).
- Attia, R., et al. Thin-layer chromatography-bioautographic method for the detection of arginase inhibitors. J Sep Sci. 43 (12), 2477-2486 (2020).
- Legerska, B., Chmelova, D., Ondrejovic, M. TLC-bioautography as a fast and cheap screening method for the detection of alpha-chymotrypsin inhibitors in crude plant extracts. J Biotechnol. 313, 11-17 (2020).
- Agatonovic-Kustrin, S., Doyle, E., Gegechkori, V., Morton, D. W. High-performance thin-layer chromatography linked with (bio)assays and FTIR-ATR spectroscopy as a method for discovery and quantification of bioactive components in native Australian plants. J Pharm Biomed Anal. 184, 113208(2020).
- Hilaire, V., et al. New method for screening anti-leishmania compounds in plants extracts by HPTLC-bioautography. J Chromatogr B. 1188, 123061(2022).
- Stankovic, J., et al. HPTLC-direct bioautography-guided isolation of isogeranic acid as the main antibacterial constituent of Artemisia santonicum essential oil. J Serb Chem Soc. 84 (12), 1355-1365 (2019).
- Su, Y., Qi, Y., Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology. 3, 195-200 (2012).
- Wedge, E., Nagle, D. A new 2D-TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J Nat Prod. 63, 1050-1054 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved