A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Radiosynthesis, Quality Control, and Small Animal Positron Emission Tomography Imaging of 68Ga-Labelled Nano Molecules
In This Article
Summary
This paper describes the radiosynthesis, formulation, quality control of a new radiolabeled probe (i.e., 68Ga-labeled nanobody NM-02), and its use for small animal PET/CT imaging in a xenograft model.
Abstract
Small animal Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) imaging techniques are crucial in preclinical cancer research, necessitating meticulous attention to radiotracer synthesis, quality assurance, and in vivo injection protocols. This study presents a comprehensive workflow tailored to enhance the robustness and reproducibility of small animal PET experiments. The synthesis process in the radiochemistry laboratory using 68Ga is detailed, highlighting stringent quality control and assurance protocols for each radiotracer production. Parameters such as concentration, molar activity, pH, and purity are rigorously monitored, aligning with standards applicable to human studies. This methodology introduces streamlined syringe preparation and a custom-designed 30G cannula for precise intravenous injections into mice. Monitoring of animal health during scanning, including temperature and heart rate, ensures their well-being throughout the procedure. Dosages for PET and SPECT scans are predetermined to balance data acquisition with minimizing radiation exposure to animals and researchers. Similarly, CT scans employ pre-programmed settings to limit radiation exposure, especially pertinent in long-term studies assessing treatment effects. By optimizing these steps, the workflow aims to standardize procedures, reduce variability, and enhance the quality of small animal PET/SPECT/CT imaging. This resource provides valuable insights for researchers seeking to improve the accuracy and reliability of preclinical investigations in molecular imaging, ultimately advancing the field.
Introduction
One topic that is of utmost relevance is research in the breast cancer field. Breast cancer remains a frequently occurring cancer, accounting for roughly 1/3rd of all cancers in women. The treatment is tailored to the biological and histological characteristics of the tumor and to the stage of the disease. The chance of survival is generally good unless the tumor has already metastasized, in which case the 5-year survival is only about 30%1. Other gynecologic cancers suffer from a similar fate, with, for example, ovarian cancer showing > 95% 5-year survival for stage 1 tumors but only 15% for metastasized stage 4 tumors2,3.
Non-invasive imaging, particularly positron emission tomography (PET), has been transforming cancer research as it offers unparalleled insights into tumor molecular aspects, such as metabolism, receptor expression, and therapeutic response4,5,6. It allows both visualization and quantification of specific metabolic areas - allowing not only to accurately diagnose, but also to monitor the effect of (new) therapies at very short time points. Indeed, PET allows evaluation of response versus non-response after 1-3 therapy cycles and does this better and faster compared to morphological changes as seen by classical computed tomography (CT) imaging7. The non-invasive nature of PET also enables longitudinal studies.
Any animal model requires maximal standardization in order to thoroughly assess the therapeutic capacity of new (radioactive) pharmaceuticals, so the emphasis has to be put on this - both in the generation of the tumor model and in the small animal PET imaging/data analysis. One could debate about the best tumor model in animals (subcutaneous inoculation or orthotopic implantation, mice, human, or syngeneic tumors, accompanied or not accompanied by routine clinical care), but that would be beyond the goal of this publication. Several models are used by us for cancer studies, and the one described here is a relatively simple subcutaneous model.
Quality control in radiochemistry is paramount for animal safety and treatment efficacy. This does not only affect the radiopharmaceutical itself but also the product formulation. There is extensive legislation on the production of radiopharmaceuticals for clinical applications8,9 (see 10 for an extensive overview of current legislation and guidelines), and several guidelines on the properties of radiopharmaceuticals for preclinical research (see 11 for an extensive overview). We produce radiopharmaceuticals both for clinical and preclinical applications, simplifying the translation from high-end quality control as found in syntheses for clinical applications into those for preclinical applications.
Our research focus is on directed theranostics, especially on human epidermal growth factor receptor 2 (HER2)-positive cancers. Hence, we develop new radiopharmaceuticals to diagnose and monitor cancer during treatment. Successful diagnostic radiopharmaceuticals are also evaluated as therapeutic compounds using different radioisotopes. The evaluation of these radiopharmaceuticals is performed at first in animal models, striving for clinical translation after promising preclinical results. In this article, we will present the protocols used, exemplified with one radiopharmaceutical, to ensure quality control and assurance, as well as standard practice for mouse intravenous injection and PET/CT scan, in order to improve the accuracy and reliability of preclinical investigations in molecular imaging. The protocol is divided into three different sections: radiochemistry (tracer synthesis and quality control), animal model generation (subcutaneous tumor model), and imaging.
Protocol
The research protocol adheres to the highest standards of animal welfare and is in strict accordance with the Animal Care Guidelines of University Hospital RWTH Aachen. We are committed to ensuring the ethical and humane treatment of all animals involved in the studies, and the procedures are reviewed and approved by the local animal ethics committee. All animal experiments were approved by a German competent authority (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, LANUV) for compliance with the Animal Protection Act, in conjunction with the regulation for the protection of animals used for experimental and other scientific purposes.
NOTE: A complete list of the equipment, materials, and reagents used throughout this study is provided in the Table of Materials. It is important to note that the handling of 68Ga should be done by pipette whenever possible and certainly avoid any metal, as the iron can vastly reduce the labeling yield. This means that needles have to be avoided until the radiochemistry procedures are completed.
1. Radiochemistry
- Radiolabeling of NM-02-DOTA-GA with 68Ga
CAUTION: The protocol involves the handling and manipulation of radioactive materials. Researchers must follow local legislation on handling radioactive materials, ALARA (as low as reasonably achievable) practices, work with lead shielding, minimize direct contact time, and maintain maximum distance at each step of radiation exposure. Experimental procedures must be monitored with radioactivity detection devices.- Elute the 68Ge/68Ga-Generator manually or automatically in 10 mL of 0.6 M HCL (1.2 GBq/10 mL). Trap the 68Ga on a PS-H+ cartridge (size M - 0.8 mL) by passing the eluate through the cartridge. Elute the PS-H+ SPE cartridge with a total of 1 mL of 4 M NaCl stepwise and slowly into a microcentrifuge tube.
- Discard the first 0.3 mL of the eluate and collect the rest 0.7 mL in another microcentrifuge tube.
- Take a new microcentrifuge tube and proceed with the radiolabeling of NM-02-DOTA-GA as stated in the manufacturer's protocol.
NOTE: The nanobody-chelator conjugate (NM-02-DOTA-GA) was previously prepared according to the protocol of the supplier. The conjugate can be stored in aliquots at -80 °C for several months. - Adjust the product's pH to around 7 using up to 5 µL of 3 M NH4OAc.
- Quality control
- Measure the activity of the concentrated sample in the microcentrifuge tube using an activity meter at the correct setting (68Ga as isotope; 1 mL syringe, 0.1 mL for geometry). The radiochemical yield (decay-corrected) should be at least 40%.
- Determine the pH with a pH test strip (0-14) by spotting a sufficient amount on the pH test strip (10-15 µL).
- Thin layer chromatography (TLC)
- Spot 1 µL of the radiolabeling reaction mixture on a silica-impregnated TLC strip. Allow the aliquot to dry for about 1 min. Run the TLC using citric acid as a mobile phase, prefilled for about 2-3 mm, in a closed TLC chamber. Remove the TLC strip from the chamber when the liquid has reached the top of the strip.
- Place the TLC strip on a radio-TLC scanner and measure it according to the manufacturer's protocol. The product should appear at the origin (Rf < 0.2), while free 68Ga cations will elute with the solvent front (Rf > 0.9).
- Calculate the radiolabeling yield of the reaction by using the radio-chromatogram, dividing the integrated area under the curve (AUC) from Rf 0.0-0.2 by the total AUC (Rf 0.0-1.0), and multiplying by 100.
- High performance liquid chromatography (HPLC)
- Make sure the HPLC has been running for at least 10 min using the correct solvent in order for all lines to be flooded and adjust the setting for the measurement. Use the isocratic flow of a 50:50 mixture of acetonitrile (solvent A) and a mixture of 0.9% NaCl solution (980 mL) combined with trifluoracetic acid (1 mL) and citrate solution (20 mL) as solvent B. Measure the UV absorbance at 280 nm.
- Take 5 µL of the product and dilute it with 15 µL of 0.9% NaCl. Inject 15 µL of the mixture in the analytical radio-HPLC (5 µm SEC-s2000 154 Å column; flow rate: 2 mL/min).
- Use the isocratic flow of a 50:50 mixture of acetonitrile (solvent A) and a mixture of 0.9% NaCl solution (980 mL) combined with trifluoracetic acid (1 mL) and citrate solution (20 mL) as solvent B. Measure the UV absorbance at 280 nm. There is a minor shift in retention times between the UV and gamma channels; this is due to the design of the HPLC (the gamma signal is installed in series after the UV detector).
- Determine the radiochemical purity by integrating the peaks in the gamma channel. The product should be in the integration range that was determined previously by measuring the non-radiolabeled nanobody. In addition, integrate all other peaks in the gamma channel and evaluate them as impurities. The purity should be >95%.
- Endotoxin
- Prepare a 1:20 dilution of the product with a total volume of 150 µL using (certified) endotoxin-free water to dilute.
- Insert a disposable endotoxin cartridge, which is commercially prefilled with all the necessary reagents, in the endotoxin testing reader and load 25 µL of the diluted product in each cartridge chamber.
- Press Play. The PTS has internal positive and negative controls and will be included in the final report. The test takes about 15 min. The product is considered endotoxin-free if the endotoxin content is < 8.7 EU/mL at Vmax = 20 mL.
- Retrospective sterility
- After injection and collection of the quality sample, store the radiopharmaceutical in a sterile vial in the refrigerator at 4 °C.
- Hand over the sample to a certified institute for analyzing sterility (either in-house or an external company) if the exemption limit of 105 Bq or 10 Bq/g is not exceeded in order to perform a sterility test according to12.
- Syringe preparation (one per animal)
- Measure the activity of the product using an activity meter at the correct setting (68Ga as isotope; 1 mL syringe, 0.1 mL for geometry).
- Take 15 MBq of the product (around 25 µL) in a microcentrifuge tube and fill the volume with 0.9% NaCl to 75 µL.
- Add 50 µL to the syringe and measure it using an activity meter at the correct setting (68Ga as isotope; 1 mL syringe, 0.1 mL for geometry). The syringe should contain 10 MBq of the product.
NOTE: As soon as the 68Ga-labeled nanobody NM-02 is formed, plastic pipette tips are no longer necessary when handling the 68Ga. From here on, whenever the protocol mentions handling a liquid, use an appropriate syringe (1 mL) and needle (27-30G).
2. Animal model generation
- Animal housing
- Use female immunodeficient Rj: ATHYM-Foxn1nu/nu mice at 6-8 weeks of age (from a commercial supplier) for developing subcutaneous tumors. Upon arrival, house all mice directly in the animal room and give at least 1 week post transport for acclimatization.
- House the animals under a 12 h light/12 h dark cycle and provide free access to food and water. Keep the room temperature and relative humidity between 20-25 °C and 45%-65%, respectively.
- Preparation of cells for implantation
- Start the cell culture with 1 x 106 SKOV-3 cells and add 25 mL of pre-warmed DMEM/F12 media enriched with 10% FBS and 1% penicillin/streptomycin in a T175 cell culture flask.
- Cultivate the cells in an incubator at 37 °C and 5% CO2 and replace the complete medium at least 2x a week.
- At 1 week or, at the latest 1 day before cell implantation, take 20 µL of medium from the cell culture flask to test for mycoplasma13. Use only mycoplasma-negative cells for cell injection.
- On the day of cell injection, aspirate the complete medium and pipette 5 mL of PBS into the bottle. Swirl the bottle back and forth several times to wash off the remaining medium.
- Add 3 mL of trypsin to the flask and incubate it for 4 min in the incubator at 37 °C and 5% CO2 to dissociate the cells. Check under the microscope with a 40x magnification if the cells detached from the cell culture flask.
- Add 7 mL of medium to neutralize the trypsin activity and transfer the cell suspension to a 50 mL centrifuge tube.
- Centrifuge the cells at 150 x g for 5 min (at room temperature) to get a cell pellet. Remove the supernatant and add 1 mL of medium to resuspend the cells.
- Take 12 µL of the cell suspension in a microcentrifuge tube and add an equal amount of trypan blue. Load the hemocytometer chamber with 10 µL of the carefully mixed cell suspension.
- Count the unstained, viable cells within the defined area and calculate the cell number. Adjust the cell concentration to 5 x 107 cells/mL media and carefully mix the cell suspension with 1 mL of basement membrane matrix.
- Take 5 x 106 cells in 0.2 mL of cell media with 50% basement membrane matrix in a 1 mL Insulin syringe for each mouse. Keep the syringe on ice until injection to prevent the solidification of the basement membrane matrix.
NOTE: If a mouse weighs less than 20 g, inject only 150 µL (for 15-20 g) or 100 µL (for 10-15 g) of the mixture. For subcutaneous applications, care is taken to ensure that the maximum volume of 10 mL/kg body weight recommended by the GV-SOLAS is not exceeded14.
- Inoculation and follow-up
- Inject the cell suspension subcutaneously into the flank of the awake animal by fixing the neck and tail with one hand. Usually, we prefer injections in the left flank.
- Wait for 10−15 s for the basement membrane matrix to harden, then remove the needle.
- Monitor the tumor growth daily post-implantation using a digital caliper to measure two perpendicular tumor axes.
- Using those measurements, assess the tumor growth using the tumor volume calculated by the formula:
VT = L × B2 × π / 6
where VT is the tumor volume in mm3, L is the length of the tumor in mm (i.e., the larger of two perpendicular axes), and B is the width of the tumor in mm (i.e., the smaller of two perpendicular axes15). This technique tends to overestimate the tumor volume as it also includes the skin. - The tumor mass influences the weight of the mouse when measured on the electronic scale. To calculate the weight of the animal corrected for the tumor mass, use the following formula:
mmouse-T = mmouse - 0.82 × VT
where mmouse-T is the mouse weight excluding the tumor mass in g, mmouse is the mouse weight according to the electronic scale in g and VT is the calculated tumor volume in mm3. The number 0.82 g/mm3 results from the mean value of a series of tumors isolated from previous animal experiments, weighed and their volume determined using the formula given. It represents an average tumor density. - Additionally, determine how large the tumor is compared to the mouse and monitor the tumor burden using the following formula:
Tumor burden [%] = (0.82 × VT) / mmouse × 100
where the tumor burden is expressed as a percentage, 0.82 × VT corresponds to the tumor mass in g (i.e., VT, the calculated tumor volume in mm3, times the mean tumor density), and mmouse is the mouse weight according to the scale in g. - When the animals reach a tumor volume of more than 200 mm3, schedule them for non-invasive PET/CT or SPECT/CT, depending on the experiment.
3. Imaging
- Syringe preparation (custom-made system)
NOTE: The mouse tail is small; therefore, the use of a 27G cannula or smaller is required (larger numbers correspond to smaller needles). In the case of female mice, the only accessible veins for bolus injections are the three tail veins. Anatomically, the dorsal tail vein is closer to the vertebral column compared to the two lateral veins; do not inject in this vein to avoid iatrogenic bone scratching. Based on experience, using a 30G cannula provides the best results as smaller veins may also be accessed. Since 30G catheters are not available for purchase, this type needs to be made with care by the researcher.- Prepare the syringe with the radioactive tracer depending on the planned route of administration. For intravenous injections, ensure that a maximum volume of 5 mL/kg body weight is administered for a 25 g mouse; this means a maximum injected volume of 125 µL.
- To prepare a 30G catheter, firstly, use any 30G needle and remove the metallic needle shaft, either with metal scissors or by bending it away from the plastic.
- Manually insert a 0.3 mm diameter PE10 tube into the part of the shaft that was removed from the hub. Care must be taken to avoid perforating the tube.
NOTE: Other types of plastic may also be used; however, PE10 offers the best balance between wall rigidity and flexibility.
- Intravenous injection
NOTE: Intravenous injections can also be done in awake animals; however, if this is not required, the animals must be injected under isoflurane sedation to reduce the burden on the research animal.- Fill the 30G catheter with 0.9% NaCl buffer or any other desired buffer.
- Place the mouse in an induction chamber and continuously fill it with a mixture of oxygen (or medical-grade pressurized air) and gaseous isoflurane until the animal loses the righting reflex. For induction, use 3.5%-5% isoflurane with a flow of oxygen or air of 0.8 L/min.
- Immediately place the sedated mouse in an initial ventral position on a heating mat (37-38 °C) while still maintaining the isoflurane sedation through a nose cone. Check the mice frequently for signs of overheating (e.g., excessive stretching, rapid breathing, redness of the skin). For maintenance of the anesthesia, use 1.8%-2.5% isoflurane with a flow of oxygen or air of 0.8 L/min.
- Apply an ophthalmic ointment to the eyes of the animal to prevent drying during sedation. Inspect both lateral tail veins and select the best one for injection. Visibility and diameter of the vein must be taken into consideration. For best results, slightly rotate the tail so that the selected vein comes in contact with the heating mat and let the vein dilate for 1 min.
- Turn the entire mouse onto the lateral position so that the selected vein comes upwards. Fixate the tip and base of the tail with tape.
- Take the 30G catheter with the dominant hand and gently place the index finger of the non-dominant hand on the selected vein to create a backup of blood inside. At the site of the backup, the vein will dilate, forming a small hump.
- With the dominant hand, place the 30G catheter parallel to the tail facing the hump on the vein. Move the dominant hand forward. Most of the time, this technique ensures a direct venipuncture.
- Once inside the vein, blood reflux becomes visible in the plastic tube. Fixate the plastic tube to ensure the needle will not move inside the vein during injection. At the free end of the tube, insert the tracer syringe connected to a 30G needle.
NOTE: When a dynamic distribution of the tracer needs to be assessed, the following points need to be performed after the animal is placed on the PET scanner bed and the PET is initiated. - Slowly inject the content of the tracer syringe for at least 10 s. While keeping the 30G needle still connected to the 30G catheter system, remove only the tracer syringe and connect a syringe with 0.9% NaCl buffer (or any other desired buffer) to flush the catheter. Monitor the breathing continuously during the injection procedure and adjust the isoflurane concentration to maintain roughly 1 breath per second.
- Remove the 30G catheter from the tail vein and use a sterile cotton compress to stop the bleeding.
- To measure the leftover activity, measure the 30G catheter, the buffer syringe, the empty tracer syringe, and the cotton compress, as all of them contain small amounts of tracer that did not reach the animal.
- PET/CT acquisition
NOTE: For 68Ga-labeled nanobody NM-02, all animals are imaged with a small animal PET /CT system.- PET acquisition
- Place the sedated mouse in a ventral position on the animal bed and attach it to the PET scanner. Switch the isoflurane flow to the nose cone on the PET and keep it at 1.5%-2.5% isoflurane in medical air at 0.8 L/min so that the breathing rate of the animal during the scan is between 75 and 50 respirations per min.
- Fix the mouse behind its neck to the animal bed with medical tape. If necessary, apply an ophthalmic ointment once again to the mouse's eyes to prevent drying during sedation.
- Turn on the heating pad in the animal bed to about 80% power, which is the equivalent of about 30 °C.
- Set the acquisition time to 45 min and select the Region of Interest that will be scanned. Select 68Ga as the study isotope and start the scan. Enter the amount of injected activity, the time of injection, and the time of measurement of the empty syringe. One PET bed position is 160 mm, which is long enough for a full-body mouse scan.
- When a dynamic distribution of the tracer needs to be assessed, first start the PET scan, wait for 30 s for acquisition, and only then proceed with steps 3.2.9-3.2.10. Leave the catheter in the mouse with the buffer syringe attached to avoid further blood loss. The catheter may be disconnected at the end of the PET, before the CT acquisition to avoid artifacts.
- CT acquisition
- Disconnect the animal bed from the PET scanner and connect it immediately to the CT scanner. If required, also detach the cannula from the tail vein. After at least 30 min post-venipuncture, the site of injection is already coagulated, so no more bleeding will occur.
- Switch the isoflurane flow to the nose cone in the CT scanner.
- Select the same region of interest to be scanned for the PET imaging. Select the standard acquisition protocol: 440 µA, 50 kVp, 32 ms exposure time, and 1080° rotation in a spiral mode with 960 exposures/360°; the duration of the CT scan is approximately 7 min for a full body mouse scan.
- Control the respiration rate and turn on the heating pad in the animal bed. Monitor the breathing continuously during the scans and adjust the Isoflurane concentration to maintain a breathing rate between 75 and 50 breaths per minute.
- PET acquisition
4. Post imaging animal care
- At the end of the scans, the animal requires at least 15 minutes to wake up. To avoid littermate aggression, cage the animal alone for about 15 min or until it is able to walk on its own until placing it back in its original housing cage. During this time, use infrared light to avoid hypothermia.
- Initially, place the animal on its left side (secure position) to avoid a hemodynamic shock. Let the ophthalmic ointment remain, as it will be removed by the animal itself once it is awake.
5. PET/CT reconstruction
- Perform PET reconstruction using a 3-dimensional ordered-subset expectation maximization (i.e., OSEM-3D with 30 iterations) with an energy window of ± 15% from a peak of 511 keV to an isometric voxel size of 0.4 mm in a 192 × 192 × 384 matrix. Apply CT-based corrections (i.e., attenuation correction, scatter correction, dead time, and random events) to the PET reconstruction.
- In case a dynamic distribution of the tracer needs to be assessed, apply a multi-frame reconstruction. As an example, the following frames can be used for a 45 min scan: 4 x 30 s, 3 x 1 min, 5 x 2 min, 2 x 5 min, and 2 x 10 min.
- Reconstruct CT standard acquisition protocols using an iterative reconstruction algorithm (i.e., ISRA) reconstruction process to an isometric voxel size of 0.2 mm in a 200 × 200 × 425 matrix. Using vendor software, convert CT values into Hounsfield units (HU) using the formula:
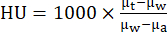
where µw is the linear attenuation coefficient of water, µa is the linear attenuation coefficient of air and µt is the linear attenuation coefficient of the tissue. - PET and CT images were automatically aligned after reconstruction using the matrix generated from a capillary phantom scan. Archive all reconstructed data as well as transfer them to a database server using image analysis software as needed.
6. Image processing and analysis
NOTE: The co-registered PET/CT images are further used for quantification within the database server of an image analysis software, where each hybrid scan is saved as a subject.
- Identify the tumor based on the CT and select this as the region of interest. Manually mask the tumor base on CT.
- To achieve user independent segmentation, threshold the newly formed tumor mask on PET or SPECT. For PET use a minimal threshold of 1.0 standardized uptake value (SUV). For SPECT, use a minimal threshold higher than the mean blood pool uptake as measured in the heart or terminal abdominal aorta.
- Record the mean, maximum, the average of the hottest 10 voxels (i.e., hot average 10) and the total uptake of the newly generated volumes of interest.
- Use those values to generate SUV and target-to-background (TBR) values, which will be further used for statistical analysis.
Results
One of the most important aspects of quality control of a radiopharmaceutical is by means of HPLC, as this shows not only the chemical and radiochemical purity (98.2% in this case) but also allows to prove the identity of the radiopharmaceutical by comparing the elution time and peak shape to that of a non-radioactive reference compound. This reference compound is, in this case, an unlabeled nanobody, proven to be the correct compound by classical techniques such as mass spectrometry or nuclear magnetic resonance. These ...
Discussion
Radiosynthesis
The radiosynthesis described here is typical for a new 68Ga-labeled compound - short synthesis time, with emphasis on suitable pH and avoiding metals whenever possible. For this, it is important to strictly follow the order in which the components are added. In any case, the pH value of the 68Ga solution must first be adjusted to pH 4 with 3 M NH4OAc; otherwise, the nanobody may degrade if the pH is too acidic. The general concepts of 68Ga-...
Disclosures
FMM is a medical advisor for NanoMab Technology Ltd. and Advanced Accelerator Applications (AAA) GmbH. He has recently received institutional grants from NanoMab Technology Ltd., Siemens, and GE Precision Healthcare LLC. Furthermore, he has an interventional research contract with CURIUM.
Acknowledgements
The authors are grateful to Susanne Allekotte for her technical support.
Materials
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Activity meter ISOMED 2010 | Nuviatech Healthcare | - | |
| Centrifuge MIKRO 185 | Andreas Hettich GmbH & Co. KG | 1203 | |
| Endotoxin testing Endosafe nexgen-PTS | Charles River | - | |
| Heating block NANOCOLOR VARIO C2 | Macherey-Nagel | 919350 | |
| HPLC system, including radio detector | Knauer & Raytest | - | |
| Image analysis software Pmod 4.4 | PMOD Technologies LLC | - | |
| Small animal PET/CT system β-CUBE and X-CUBE | Molecubes NV | - | |
| TLC MiniGITA* | Elysia-Raytest | - | |
| Materials | |||
| 0.3 mm diameter PE10 tube | fisher scientific | 22-204008 | |
| 30G needle | B|Braun | 4656300 | |
| Centrifugal filter; 10 kDa MWCO, 0.5 mL | Millipore | UFC501008 | |
| Chromatography paper strip iTLC-SG | Agilent Technologies | SGI0001 | |
| Endotoxin Cartridge, 0.05 EU/ml sensitivity | Charles River | PTS-2005 | |
| HPLC Column Biosep SEC-s2000 | Phenomenex | - | |
| Microcentrifuge tube (1.5 mL) | Eppendorf | 0030125150 | |
| pH strip 0.0 - 6.0 | Merck KGaA | 109531 | |
| pH strip 0-14 | Merck KGaA | 109535 | |
| PS-H+ SPE cartridge | Macherey Nagel GmbH & Co. KG | 731861 | |
| Sterile vial 10 mL | ALK Life Science Solutions | SEV100 | |
| Reagents | |||
| 68Ge/68Ga-Generator | NRF-iThembaLABS | - | |
| Ammoniumacetate | Merck KGaA | 101116 | |
| Citric acid | Merck KGaA | 100241 | |
| Hydrochloric acid | Merck KGaA | 320331 | |
| NaCl | Merck KGaA | S9888 | |
| Nanobody NM-02 | Radiopharm Theranostics | - | |
| P-SCN-Bz-DOTA-GA | CheMatech | C115 | |
| Trifluoracetic acid | Merck KGaA | T6508 | |
| Ultrapure water | Merck KGaA | 101262 |
References
- American Cancer Society. . Cancer facts & figures 2024. , (2024).
- Cancer Research UK. . Ovarian cancer survival. , (2021).
- Siegel, R. L., Giaquinto, A. N., Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. 74 (1), 12-49 (2024).
- Altunay, B., et al. Her2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 48 (5), 1371-1389 (2021).
- Altunay, B., Morgenroth, A., Mottaghy, F. M. Use of radionuclide-based imaging methods in breast cancer. Semin Nucl Med. 52 (5), 561-573 (2022).
- Mcgale, J., et al. Pet/ct and spect/ct imaging of her2-positive breast cancer. J Clin Med. 12 (15), 4882 (2023).
- Zhang-Yin, J. State of the art in 2022 pet/ct in breast cancer: A review. J Clin Med. 12 (3), 968 (2023).
- USFD Administration. . Pet drug products - current good manufacturing practice (cgmp). , (2018).
- . . Official Journal of the European Union, L238. 60, (2017).
- Korde, A., et al. Position paper to facilitate patient access to radiopharmaceuticals: Considerations for a suitable pharmaceutical regulatory framework. EJNMMI Radiopharm Chem. 9 (1), 2 (2024).
- Korde, A., et al. Practical considerations for navigating the regulatory landscape of non-clinical studies for clinical translation of radiopharmaceuticals. EJNMMI Radiopharm Chem. 7 (1), 18 (2022).
- . . European Pharmacopeia. , (2023).
- Volokhov, D. V., Graham, L. J., Brorson, K. A., Chizhikov, V. E. Mycoplasma testing of cell substrates and biologics: Review of alternative non-microbiological techniques. Mol Cell Probes. 25 (2-3), 69-77 (2011).
- Dülsner, A., et al. Technical information from the Committee for Animal Welfare Officers (GV-SOLAS) and Working Group 4 in the TVT Recommendation on substance administration to laboratory animals. GV-SOLAS. , (2017).
- . derived xenograft (pdx) protocols at the jackson laboratory Available from: https://tumor.informatics.jax.org/mtbwi/live/www/html/SOCHelp.html (2020)
- Velikyan, I. 68ga-based radiopharmaceuticals: Production and application relationship. Molecules. 20 (7), 12913-12943 (2015).
- Percie Du Sert, N., et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 18 (7), e3000411 (2020).
- Smith, A. J., Clutton, R. E., Lilley, E., Hansen, K. E. A., Brattelid, T. Prepare: Guidelines for planning animal research and testing. Lab Anim. 52 (2), 135-141 (2018).
- Landesamt Für Natur UUVN. . Tierversuche. , (2024).
- Herrmann, D., et al. Statistical controversies in clinical research: Publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Ann Oncol. 28 (5), 931-937 (2017).
- Johnsen, E., et al. A critical evaluation of amicon ultra centrifugal filters for separating proteins, drugs and nanoparticles in biosamples. J Pharm Biomed Anal. 120, 106-111 (2016).
- . TVT-Veröffentlichungen zum Download Available from: https://www.tierschutz-tvt.de/alle-merkblaetter-und-stellungnahmen/ (2024)
- Bedard, P. L., Hansen, A. R., Ratain, M. J., Siu, L. L. Tumour heterogeneity in the clinic. Nature. 501 (7467), 355-364 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved