Method Article
A Mouse Model for Vascular Cognitive Impairment and Dementia Based on Needle-guided Asymmetric Bilateral Common Carotid Artery Stenosis
In This Article
Summary
The needle method of asymmetric bilateral common carotid artery stenosis is proposed to create a mouse model for vascular cognitive impairment and dementia. It results in longer-term outcomes compared to previously established models and is compatible with live MRI. Visual representation demonstrating the procedure provides guidance for mastering the surgery.
Abstract
Vascular cognitive impairment and dementia (VCID) results from vascular brain injury. Given VCID's high incidence, which is expected to continue rising as the population ages, it is critical to establish a robust animal model for the disease. This paper presents a novel method of creating a mouse model of VCID that is based on asymmetric bilateral common carotid artery stenosis, which mimics human chronic cerebral hypoperfusion caused by carotid atherosclerosis.
Briefly, common carotid arteries (CCAs) are ligated to different gauge needles (32 G for the right CCA and 34 G for the left CCA) using 7-0 silk sutures followed by immediate needle removal. The remaining suture rings cause persistent blood flow reduction and long-term cognitive impairment associated with white matter injury, microinfarcts, and reactive gliosis, thus closely mimicking the pathogenesis of VCID. Importantly, in this needle model, the clinical representations do not revert with time, providing reliable long-term cognitive impairment. Moreover, the survival rate 24 weeks post surgery was 81.6%, which is higher compared to the other established models of VCID with a similar level of blood flow reduction.
Additional advantages include low material cost and compatibility with MRI to monitor brain injury in live animals since no metal is implanted. The main challenge in employing the needle model of VCID is the requirement for developing advanced surgical skills since mouse CCAs are less than 0.6 mm in diameter and are very fragile. High-quality visual representation of the surgery will thus help researchers to master this technique and advance our understanding of VCID, potentially leading to the development of novel therapeutic modalities to decrease the devastating cognitive decline associated with VCID.
Introduction
Vascular cognitive impairment and dementia (VCID) is the second leading cause of cognitive decline. Despite the undeniable progress that has been made toward understanding VCID pathogenesis and its risk factors, the mechanism of how neurovascular dysfunction contributes to the decline of cognitive ability remains vague. A number of rodent models of various complexity have been established to induce cerebral ischemia to mimic the clinical representations of human VCID1. Some of these models are based on creating transient cerebral hypoperfusion; however, most of them are generated by inducing chronic cerebral hypoperfusion, the main mechanism that leads to VCID in human patients2.
Chronic cerebral hypoperfusion can be introduced using either bilateral carotid artery occlusion (BCAO), which causes severe, but often fatal results, or bilateral carotid artery stenosis (BCAS). BCAS is usually performed using one of two methods: by placing identical microcoils around both CCAs, resulting in symmetric stenosis3; or by implanting an ameroid constrictor and a microcoil around the left and right CCAs, respectively, causing gradual occlusion and ~50% blood flow reduction on the left and right CCAs, correspondingly4. The drawbacks of both methods are high mortality rate if stenosis is too severe or if the CCA is occluded and incompatibility with live-animal MRI scan due to the presence of metal in the body. A few genetic mouse models have also been established1,5,6,7,8. Additional options include cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy mouse models9,10. Nevertheless, none of the proposed models mimics the full range of ischemic damage that is presented in human patients, and therefore the search for updated VCID models continues.
This paper presents a novel surgical method of inducing asymmetric bilateral common carotid artery stenosis (ABCS) in mice, where CCA stenosis is performed using silk sutures and is controlled by ligating CCAs to needles of various diameters followed by immediate needle removal11. As a result, suture rings of precise diameters are permanently left on the CCAs to ensure chronic stenosis. The benefit of using ABCS over a symmetric method is that moderate hypoperfusion on the right ensures better survival while more pronounced hypoperfusion on the left assures long-term neurological and pathological representations. This needle model has several advantages over the traditional BCAS models11 such as persistent results, lower mortality, ultra-low cost, flexibility, and the possibility to use special analytic approaches.
To elaborate on these advantages, three ligations cause a fragment of CCA stenosis rather than focal point stenosis, leading to persistent hypoperfusion, white matter injury, and cognitive decline in ~90% of mice. The mortality of the needle mice was ~17%, lower than that of Hattori's ameroid restrictor/microcoil model4, which has ~30% mortality over 16 weeks based on our experience. Each BCAS model typically costs around $100 due to the expensive microcoils or ameroid restrictors, while the needle model costs only about $1 per mouse. Moreover, the gauge of the needles could be modified depending on the research-specific requirements for the blood flow restriction on either side. In the variation presented in the current paper, the needle model mimics the pathophysiology of severe carotid stenosis, caused by unilateral permanent stenosis without occlusion, which is the most common representation of the disease in clinic11. Further, ameroid restrictors and microcoils that are used in traditional BCAS models are made of metal, which can cause significant artefacts if in vivo MRI is performed, even though the metal is implanted not in the brain but in the chest. It might be difficult to predict how exactly the presence of metal would affect the imaging.
Generally, in vivo MRI that is performed after microcoil implantation is usually simple anatomical imaging, not suitable for quantitative analysis of multiple insults, which is highly desirable for VCID research. In contrast, the needle model presented here uses only silk sutures and is fully compatible with any kind of in vivo MRI. This is significant for two reasons: (1) MRI is extremely sensitive to small brain lesions, microbleeds, or superficial siderosis12, thus is preferred over the other methods of analysis, such as CT scan (2) in vivo MRI should be preferred over the ex vivo MRI, as VCID research can undoubtedly benefit from tracking the dynamics of lesion progression/healing, especially in response to the proposed novel treatments. Additionally, functional (fMRI) can be performed in the needle model to provide critical insights into the integrity of neurovascular coupling in response to cerebral hypoperfusion. Thus, the possibility of using in vivo MRI opens an avenue for in-depth analysis of the intricate correlation between the size and the location of the lesions and cognitive function, as well as neurovascular coupling, especially in pharmacodynamics studies.
Protocol
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sterile techniques must be maintained in all survival surgeries. Twelve-week-old male C57BL/6J mice with body weight 25-30 g were used in the presented experiments.
1. Preparation of the materials and working space
- Prepare the needle fragments (~4 mm long; 32 G for the right CCA and 34 G for the left CCA). Slightly blunt the sharp end of the needles by carefully tapping the sharp end against a hard surface and carefully separate the needles from the plastic pieces using a needle holder. Precut 7-0 silk sutures into 1-2 cm long pieces.
- Sterilize surgical instruments, needle fragments, and sutures by autoclaving or any other appropriate method.
2. Performing the surgery
- Weigh the mouse and induce anesthesia by placing the mouse for 2-3 min in a chamber ventilated with 3% isoflurane in a mixture of 25% O2 and 72% N2O.
- Place the mouse (ventral side up) on a heating pad covered by a sterile surgical sheet to maintain a constant body temperature (37 °C) and secure a face mask for ventilation with 1% isoflurane in a mixture of 25% O2 and 74% N2O. Secure the mouse's limbs with adhesive tape.
- Shave the fur on the neck with an electric shaver. Clean the fine hair with adhesive tape or a keyboard vacuum.
- Disinfect the surgical site with Betadine Solution (10% iodine). Deiodize the skin with 70% ethanol. Repeat dinsinfection 3x. Ensure that the level of anesthesia is adequate by a lack of response to a firm toe pinch (pedal reflex).
- Drape the surgical area. Then, make a vertical midline incision along the trachea and separate the bilateral thyroid glands with micro forceps. Pull the skin and tissue away with sterile small skin retractors.
- Under the microscope, use angled tweezers to carefully expose and blunt dissect one of the CCAs from the vagus nerve and the sheath. Use sterile water or phosphate-buffered saline (PBS) to wet the incision site if the CCA tends to stick to the forceps. Place a small plastic syringe under the neck to support posture if required.
NOTE: Take extra care not to rupture the CCA. - Thread three precut silk suture fragments (size 7-0) under the CCA (1 mm apart) using angled tweezers.
- Draft a very loose double knot around the CCA on one of the suture fragments.
NOTE: Wetting the surgical site and the sutures with sterile water or PBS might help with drafting a knot. - Place a fragment of needle (32 G for the right CCA and 34 G for the left CCA) parallel to the CCA, inside the loose knot (Figure 1A). Carefully tighten the primary knot around both the needle and the CCA until no blood flow is observed and secure it with a secondary knot. Immediately pull out the needle to restore partial blood flow and trim the ends of the suture.
NOTE: The surgeon must be very careful trimming the suture ends after the knots are completed. Cutting too close to the knot might result in loosening the knot. Additionally, it is critical to observe the CCA after removing the needle to make sure that the blood flow is still enabled downstream of the knot. If the CCA looks very pale after needle removal, it means that the CCA is overcompressed. - Repeat steps 2.8-2.9 for the 2nd and 3rd suture threads on the same CCA, approximately 1 mm apart from each other (Figure 1B).
- Repeat steps 2.6-2.10 with the opposite CCA using a 32 G needle fragment.
- Carefully examine whether both CCAs are efficiently ligated: confirm that all three suture bands are securely knotted in place but are not so tight as to completely block the blood flow (check that the CCAs are not pale downstream of the knots). Check that the three suture bands on each CCA are approximately 1 mm apart from each other.
- Close the skin using sterile monofilament sutures.
- Immediately after the surgery, inject 100-150 µL of ketoprofen (1 mg/mL stock; 5 mg/kg body weight) intraperitoneally to relieve postoperative pain. Repeat this injection 24 h and 48 h after the surgery.
- Place the mouse on a homeothermic blanket at 37 °C for continuous monitoring for 2 h prior to returning the animal to the animal facility.
NOTE: For sham procedure, steps 2.7-2.12 should be omitted.
3. Validation of the model
NOTE: Brain injury was analyzed with in vivo MRI and further verified with Luxol fast blue (LFB) staining and behavioral tests.
- Imaging of cerebral blood perfusion.
- Anesthetize the mouse using 1-1.5% isoflurane. Sterilize the surgical site with Betadine Solution (10% iodine). Then, deiodize the skin with 70% ethanol.
- Secure the skull of the animal in a stereotactic frame. Make a midline saggital incision in the scalp from frontal to occipital bone to expose the skull and clean the skull surface with sterile saline.
- Place a charged-coupled device camera 10 cm above the skull using a two-dimensional laser speckle system. Put a probe holder over the craniectomy site and secure it firmly.
- Take blood perfusion images 5 min before surgery and immediately after needle release or 7, 14, 21, 28, 35, and 42 days after surgery.
- Close the skin using sterile monofilament sutures. Inject 100-150 µL of ketoprofen (1 mg/mL stock; 5 mg/kg body weight) intraperitoneally to relieve postoperative pain. Repeat this injection 24 h and 48 h.
- In vivo MRI
- Anesthetize the mice using 1-1.5% isoflurane, with respiration continuously monitored and temperature maintained at 37 °C with warm air during image acquisition.
- Perform in vivo MRI using a 9.4T scanner, an 86 mm Tx coil and 4-channel mouse brain receiver array, running the associated software. Following positioning and pilot scans, acquire T2-weighted images (T2WI) using a Rapid Acquisition with Relaxation Enhancement (RARE) sequence, with the following parameters: Echo Time/Repetition Time (TE/TR) = 40/4,000 ms, averages = 8,256 × 256 matrix, 16 slices with a 0.5 mm slice thickness, a RARE factor = 4, and a field of view (FOV) of 20 x 20 mm.
- Collect Diffusion Tensor Imaging (DTI) data using using an echo planar imaging (EPI)-DTI imaging sequence using the same geometry and parameters as the T2WI with the following exceptions: TR/TE = 2,300/22 ms, acquisition matrix = 128 x 128, 2 segments, 5 A0 images, and 30 noncolinear diffusion images, Δ/δ = 10/3 ms, and a b-value = 1,000 s/mm2.
- Analyze DTI data using DSI Studio software (http://dsistudio.labsolver.org/), looking for differences in diffusion scalar parameters (Fractional Anisotropy (FA), Mean Diffusivity (MD), Axial Diffusivity (AD) and Radial Diffusivity (RD)). Draw regions of interest (ROIs) for the corpus callosum (CC), external capsule (EC), internal capsule (IC), Fimbria, anterior commissure (AC), cingulum (Cing), hippocampus (hippo), Cortex (C), and striatum (Str) from both hemispheres.
- Luxol fast blue (LFB) staining
- Prepare brain sections: fix the brain with 4% PFA for 24 h and then immerse the brain in 30% sucrose until the brain sinks. Embed the brain in OCT compound on dry ice. Cut 20 µm-thick coronal brain slice on a sliding microtome. Store brain slices in storing solution (30% glycerol/30% ethylene glycol in PBS).
- Immerse brain sections in LFB (0.1% alcohol solution), keep at 56 °C overnight, and wash with distilled water.
- Incubate brain sections in 0.05% lithium carbonate and dehydrate through graded alcohols.
- Stain the sections with 0.5% cresyl violet for 5 min, differentiated with 70% ethanol.
- Mount the stained brain sections with mounting medium.
- Cognitive function assessment with a modified Morris water maze test
- Fill a circular tank with water (25 oC, depth 33 cm) and submerge a square ~10 x 10 cm2 plexiglass platform 1.2 cm below the water level, 31 cm from the north, east, south, or west edge of the pool.
NOTE: This platform should remain in the same place for the whole duration of testing. - Turn on a ceiling-mounted video camera. Place a mouse in the tank (do not drop-release at water level), facing the wall and starting at either northeast, southeast, southwest, or northwest. Allow the mouse to swim for a maximum of 90 s to find a submerged platform.
- Allow the mouse to remain on the submerged platform for 30 s if the platform is found. If the platform is not found, place the mouse there for 30 s.
- Let the mouse rest for 5 min and repeat the swimming trial (steps 3.4.2 - 3.4.3) 2x.
- Dry the mouse with a piece of cloth and return it back to its cage under a heating bulb.
- Repeat three swimming trials every day for 5 sequential days.
- Using video footage, calculate latency to escape (how long does it take to find the platform in each trial).
- On the last day of testing, remove the platform and record the time that the mouse spends in the quadrant, where the platform was previously located.
- Fill a circular tank with water (25 oC, depth 33 cm) and submerge a square ~10 x 10 cm2 plexiglass platform 1.2 cm below the water level, 31 cm from the north, east, south, or west edge of the pool.
Results
Long-term asymmetrical cerebral hypoperfusion
CCA blood flow was measured before and immediately after releasing the needle from the last (third) ligation, as described previously11. Blood flow was reduced by ~70% in the left CCA and ~50% in the right CCA. Cerebral blood perfusion was dynamically monitored using two-dimensional laser speckle. The surgery caused cerebral hypoperfusion in both hemispheres, with the left hemisphere being more severely affected (Figure 2). Cerebral hypoperfusion is maintained for at least 24 weeks after surgery11.
Lower mortality
The survival rate for male mice over 6 weeks was 81.6% (Figure 3); mice were more prone to death in the first week after surgery.
In vivo MRI detection of brain injury
T2-weighted images revealed hypo- or hyperintense regions in the hippocampus (Hippo), external capsule (EC), internal capsule (IC), corpus callosum (CC), and striatum (Str) in mice subjected to ABCS surgery, indicating brain injury (Figure 4A). Structural damage was observed in the directionally encoded color (DEC) maps of DTI of ABCS mice when compared with the sham (Figure 4B).
Quantitative analyses showed that ABCS mice showed significantly lower fractional anisotropy (FA) in left Hippo, IC, anterior commissure (AC), cingulum (Cg) and Fimbria (Fi) when compared to sham (P < 0.05 versus sham), indicating compromise of white matter microstructure in the left hemisphere (top left panel, Figure 4C). FA in the right Cg and Fi in ABCS mice were also reduced (P < 0.05 versus sham). FA in the left IC and Fi were significantly lower than that of the right in ABCS mice (P < 0.05, left versus right).
Similarly, significantly lower axial diffusivity (AD) was exhibited in the left EC, IC, Str, and Fi in ABCS mice as compared with sham, suggesting axonal injury (top right panel, Figure 4C (P < 0.05 versus sham)). Only the left Str showed mean diffusivity (MD) reduction in ABCS mice as compared to sham (bottom left panel, Figure 4C). Radial diffusivity (RD) difference was observed in left CC, Str, and Fi in ABCS mice (bottom right panel, Figure 4C), suggesting inflammation and increased cellularity in these regions13.
Asymmetrical brain injury in the two hemispheres and damage in the white matter regions in the left hemisphere
Brain injury was additionally analyzed with LFB staining using the procedure described previously14 (Figure 5). Lower power images showed lighter blue staining in EC and Str, suggesting demyelination in these regions. High-definition images showed that sham mice displayed well-organized and myelinated axons with lineal-oriented oligodendrocytes in EC; however, axons disappeared and pervasive, blue-stained cells were observed in EC in ABCS mice. Sham mice showed deep blue-stained and well-organized fiber bundle structures in Str. However, fiber bundles stained lighter and smaller, and their integrity was compromised; some fiber bundles were vacuolated in ABCS mice. Fiber bundles were distorted, and the inter-bundle matrix was significantly thickened in AC in ABCS mice. In summary, ABCS surgery causes axonal demyelination and damage; white matter damage occurs mainly in the left hemisphere.
Learning and memory dysfunction
The needle ABCS surgery resulted in significant learning disability, as indicated by an increased time to find a submerged platform (latency to escape) during a Morris water maze test (Figure 6). This disability pertained for at least 24 weeks after the surgery. Memory was also significantly affected, as indicated by a decreased time spent in the target quadrant after the platform has been removed.
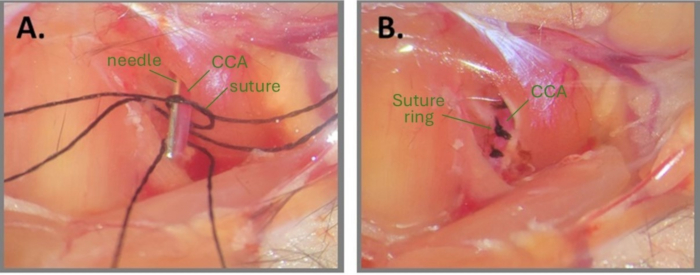
Figure 1: Steps of needle surgery to create ABCS. (A) Left CCA is exposed, and three silk sutures are threaded under the CCA. A loose knot is drafted on one of the suture fragments and the needle is positioned parallel to the CCA. (B) Three suture rings are positioned approximately 1 mm apart. Abbreviations: ABCS = asymmetric bilateral common carotid artery stenosis; CCA = common carotid artery. Please click here to view a larger version of this figure.

Figure 2: Persistent asymmetrical cerebral hypoperfusion in the needle ABCS model. Dynamic monitoring of brain blood flow after the surgery. Persistent cerebral hypoperfusion can be observed in both hemispheres, being more profound on the left. Black asterisks indicate hypoperfusion regions. Abbreviation: ABCS = asymmetric bilateral common carotid artery stenosis. Please click here to view a larger version of this figure.

Figure 3: Survival rate after needle ABCS surgery. The surgery resulted in an 81.6% survival rate over 6 weeks; death occurred mainly during the first postoperative week. Abbreviation: ABCS = asymmetric bilateral common carotid artery stenosis. Please click here to view a larger version of this figure.
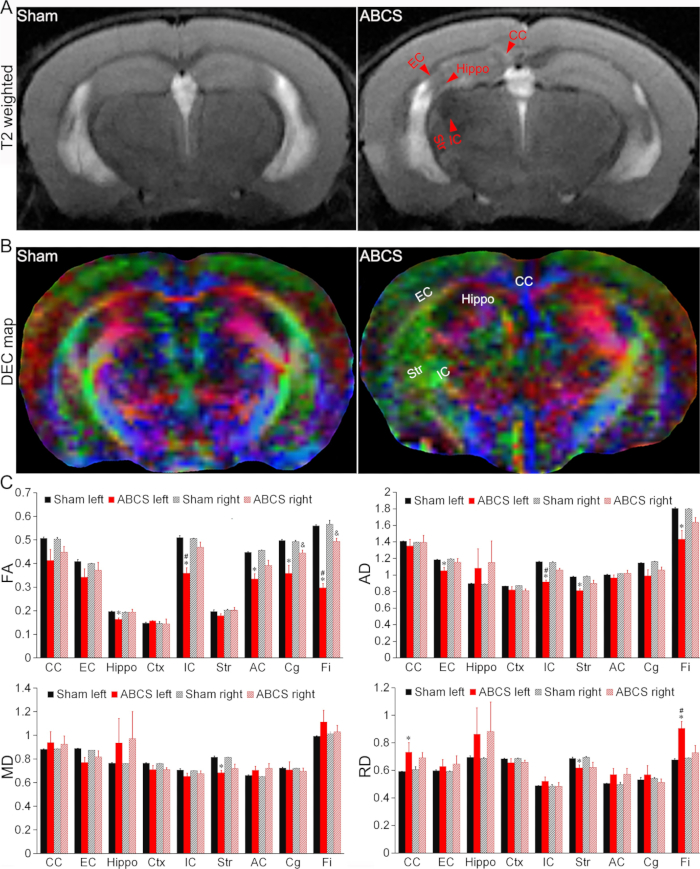
Figure 4: In vivo MRI detection of brain injury in the needle ABCS model. (A) Representative T2-weighted images from sham and ABCS mice. Red arrowheads indicate hypo- or hyperintense regions. (B) Representative DEC map of in vivo DTI 2 weeks after needle ABCS surgery. The colors indicate the directionality of the principal axis of diffusion (red = left/right, green = dorsal/ventral, and blue = rostral/caudal). (C) Quantitative analysis in sham and ABCS mice (one-way ANOVA). * represents p < 0.05 ABCS versus sham (left hemisphere). # represents p < 0.05 left versus right. & represents p < 0.05 ABCS versus sham (right hemisphere). Data are presented as mean ± SEM; n = 4 for sham, n = 4 for ABCS mice. Abbreviations: ABCS = asymmetric bilateral common carotid artery stenosis; Hippo = hippocampus; EC = external capsule; IC = internal capsule; CC = corpus callosum; Str = striatum; DEC = directionally encoded color; DTI = diffusion tensor imaging; FA = fractional anisotropy; MD = mean diffusivity; AD = axial diffusivity; RD = radial diffusivity. Please click here to view a larger version of this figure.
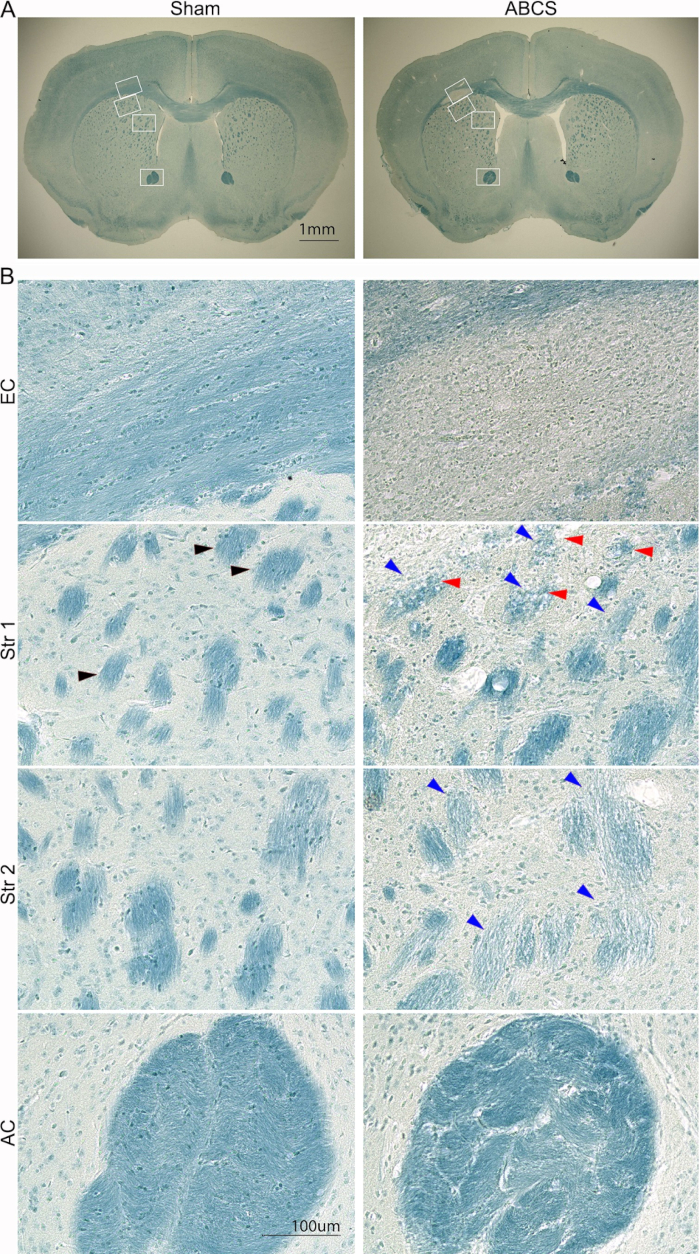
Figure 5: Representative LFB staining of brain slices 6 weeks after the needle ABCS surgery. (A) Low-power images of LFB staining from sham and ABCS mice. White boxes denote the location corresponding to higher-definition images in B. (B) High-definition images (200x) from EC (top panel) and Str locations 1 and 2 (middle panel) and AC (bottom panel). Black arrowhead shows a normal fiber bundle. Blue arrowheads point to a damaged fiber bundle. Red arrowheads denote vacuolated fibers. Scale bars = 1 mm (A), 100 µm (B). Abbreviations: ABCS = asymmetric bilateral common carotid artery stenosis; LFB = Luxol fast blue; EC = external capsule; Str = striatum; AC = anterior commissure. Please click here to view a larger version of this figure.
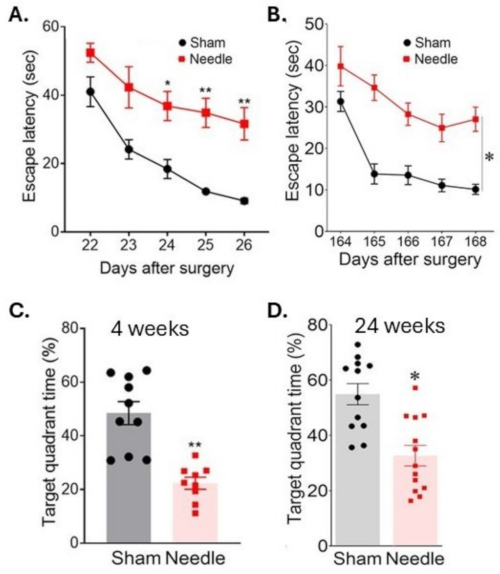
Figure 6: Spatial learning and memory assessment in water maze test. Spatial learning was assessed (A) 3 weeks and (B) 24 weeks after the surgery by measuring the time to locate the platform on consecutive days. Memory was assessed (C) 4 weeks and (D) 24 weeks after the surgery by measuring time spent in the target quadrant after the platform has been removed. The surgery resulted in long-term disruption of cognitive functions. Data were expressed as mean ± SEM, *p < 0.05 needle versus sham; **p < 0.01 versus sham; two-way ANOVA and Neuman-Keuls post hoc. n = 10 for sham, n = 10 for needle groups. This figure has been modified from Weng et al.11. Please click here to view a larger version of this figure.
Discussion
Several methods of VCID induction using asymmetric CCA stenosis have been described, and all of them share an important and critical surgical step-isolation of the CCA from the vagus nerve and the sheath and exposure of the CCA to make it accessible for stenosis. While we provide good-quality visual guidance on the surgical exposure of CCAs prior to ligation, we would also like to direct researchers to watch additional videos on CCA isolation that are available online in the context of other mouse and rat surgeries15,16,17,18. Extra care should be taken not to compress or rupture the CCA as it is the main artery that supplies the brain with oxygenated blood.
Another critical step of the surgery that needs to be mastered is drafting a loose knot and further tightening it around the CCA with the needle followed by needle removal. We highly recommend practicing this step with just the needle alone before attempting to perform it in an anesthetized mouse. This will allow for mastering delicate forceps movements and perfecting manipulations with the sutures without the risk of hurting the mouse. The knot needs to be secured well without falling apart during needle removal, tight enough to hold the needle but loose enough to enable sliding the needle away from the knotted suture using forceps.
Importantly, we found that a single ligation of each CCA is not enough to reliably decrease the blood flow and to maintain long-term cerebral hypoperfusion11. One potential explanation might be that a single ligation causes point stenosis, which is likely to cause a local increase in pressure, leading to increased velocity of blood flow to compensate for the blood flow reduction. We recommend performing three ligations ~1 mm apart from each other to create a fragment of stenosis. An additional benefit of using three ligations is that the knots serve as a proofing mechanism in case one of the knots gets loose during additional needle removal. Indeed, a fragment of stenosis caused by three separate ligations of the same CCA increases model consistency, leading to persistent cerebral hypoperfusion in about 90% of mice. Moreover, fragment stenosis mimics CCA stenosis caused by atherosclerosis in human patients more precisely compared to focal point stenosis, thus increasing the clinical relevance of the needle model.
We strongly recommend prompt removal of the needle after finishing the first ligation to make sure CCA blood flow is partially recovered, and only then move on to the second and third ligations sequentially. It is not recommended to finish all three ligations with the needle inside all three knots, as this would significantly increase the time of complete obstruction of blood flow. Typically, we do not recommend more than 1 min of complete CCA occlusion before removing the needle. This recommendation is based on the report that mice showed no signs of any functional impairment after 3 sessions of 60 s occlusion of both CCAs19. In our model, the surgeon works on one CCA at a time, which is more forgiving than the occlusion of both CCAs simultaneously, but we still recommend following this timeline to exclude any artifacts caused by prolonged occlusion rather than chronic hypoperfusion on either side.
While this needle model allows for adjusting the diameters of the knotted sutures by using needles of various diameters (based on body weight or the specific requirements of blood flow restriction), in our experience, mice had higher survival rates when hypoperfusion in the right hemisphere was moderate rather than severe. On the other hand, persistent severe hypoperfusion in the left hemisphere produced long-term pathological and neurological outcomes. Thus, we recommend performing an asymmetric ligation by using a thicker needle to ligate the right CCA (causing moderate hypoperfusion in the right hemisphere) and a thinner needle to ligate the left CCA (causing severe hypoperfusion in the left hemisphere).
Lastly, researchers should be aware that different mouse strains may produce different outcomes from the ischemic or traumatic insult, mainly due to differences in cerebral vascular anatomy20,21. Since many studies nowadays require the generation of novel transgenic mice, the background strain must be carefully considered if BCAO surgery is required at any stage of the experimental design. For example, both C57BL/6 and SV129 strains are a common background choice for generating transgenic animals for stroke research21. However, it has been well documented that C57BL/6 mice are much more sensitive to ischemia compared to the other strains tested, including SV129 mice20,21. In fact, there is evidence that the effect of the murine strain can be even more important than the effect of the technique used to induce VCID21. Thus, it becomes critically important to keep the mouse backgrounds consistent throughout all experiments that involve inducing ischemic brain injury in rodents. Importantly, researchers can evaluate the efficiency of the surgery outcome in live animals using neurological scoring systems22 with a score of 0.5 as an inclusion criterion. Brain injury can be further confirmed using Iba1 immunostaining which is very sensitive to brain damage even after minor focal insult.
In summary, it is important to remember that VCI is a complex term that unites many clinical representations and causes under the same umbrella. Therefore, researchers should always keep in mind which model should be selected based on the VCI aspects that they would like to study. There can never be a single universal model for all VCI manifestations. CCA stenosis models severely limit blood flow from the major arteries, thus mimicking patients with arteriosclerotic stenosis. The novel needle method of creating asymmetric BCAS in C57BL/6J mice is a reliable method to mimic VCID that offers several advantages over the previously reported methods (particularly high flexibility, low mortality, long-term outcomes, minimal cost, and live MRI monitoring). Due to its advantages over the other models, it can be used to further advance our knowledge of VCID progression as well as serve as a basis for screening potential therapeutic agents to cure or slow the progression of VCID. Similar to other reported methods of BCAS, the needle model requires advanced surgical skills that can be mastered with time, using this visual demonstration as a guide.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This project was supported by National Institutes of Health/NINDS grants RF1NS117509 (G. Cao) and VA Merit Review grants BX003923 and BX006454 (G. Cao).
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL syringe | BD | 309659 | IP injection of ketoprofen |
| 2.5 mm blunt retractor tips | Kent Scientific Corporation | SURGI-5016-2 | 2 needed |
| Andis Ion T-Blade Pet Trimmer | Andis | BTF3 | To shave fur |
| Betadine Solution | Avrio Health L.P. | NDC 67618-150-17 | |
| Braided silk suture, 5-0 | Teleflex Medical | 106-S | For suturing skin |
| Braided silk suture, 7-0 | Teleflex Medical | 103-S | For ligating CCAs |
| Camera | Med Associates, Inc | VID-CAM-MONO-7 | For model validation |
| cover glass | Fisherbrand | 12-541-012 | For model validation |
| cresyl violet | Thermo Scientific | C581-25 | For model validation |
| Dispensing Tip, Needle, Flex, 34 Gauge, 1/4" OAL | Jensen Global | JG34-0.25HPX | For the left CCA |
| DSIStudio | https://dsi-studio.labsolver.org; for DTI data analysis | ||
| Dumont Tweezers; Pattern #1 | Roboz Surgical Instrument Co | RS-4960 | 2 needed |
| Dumont Tweezers; Pattern #5, 45 Degree Angle | Roboz Surgical Instrument Co | RS-5058 | 2 needed |
| Ethanol | Fisher Scientific | 64-17-5 | Dilute to 70% |
| ethylene glycol | Thermo Scientific | E178-4 | For model validation |
| Eye Needle, Size #4; 3/8 Circle, Cutting Edge, 11 mm Chord Length | Roboz Surgical Instrument Co | RS-7981-4 | For suturing skin |
| E-Z Anesthesia Classic System | E-Z systems | EZ-7000 | |
| glycerol | Thermo Scientific | AAA16205AP | For model validation |
| Homeothermic Monitoring System | Harvard Bioscience | K 022258 | |
| Imaging system EVOS FL Auto | Life Technologies | AMAFD2000 | For model validation |
| Isoflurane | Covetrus | 11695-6777-2 | |
| Ketoprofen | Zoetis | 5487 | |
| Leica M320 F12 clinic and surgery microscope | Leica | M320 F12 | |
| Lidocaine and Prilocaine Cream, USP 2.5%/2.5% | Padagis | NDC 0574-2042-30 | Generic to EMLA cream |
| lithium carbonate | Thermo Scientific | L119-500 | For model validation |
| Luxol fast blue | Thermo Scientific | AC212170250 | For model validation |
| microscope slides | Fisherbrand | 12-550-403 | For model validation |
| Microtome | Leica | SM2010R | For model validation |
| Morris Water Maze with the hidden platform | Maze Engineers | https://maze.conductscience.com/portfolio/morris-water-maze/ | |
| M-Prove Portable Balance | Sartorius | AY711 | Scales for weiging the mouse |
| MRI with ParaVision 6.0.1 | Bruker | AV3HD 9.4T | For model validation |
| Multi gas flow meter | Aalborg Instruments | GMR2-010334 | for low flow rate gas blending (N2O and O2) |
| Nano Ultra Fine Pen Needles - 32G 4mm | BD | 58320883 | For the right CCA |
| O.C.T. | Thermo Scientific | 23-730-571 | For model validation |
| Paraformaldehyde, 4% | Thermo Scientific | J61899.AK | For model validation |
| PBS | Thermo Scientific | BP399500 | For model validation |
| PeriCam PSI System with Aperiflux probe holder | Perimed Inc | PeriCam PSI HR | For model validation |
| permount | Thermo Scientific | SP15-100 | For model validation |
| Round Handle Forceps; Micro Suturing With Tying Platform; Curved | Roboz Surgical Instrument Co | RS-5264 | To help with cutting and suturing the skin |
| Small Animal Heat Lamp, 75 Watt | Morganville Scientific | HL0100 | For model validation |
| Small cotton-tipped applicators | Fisher Scientific | 23-400-118 | |
| Spring Scissors - 8mm Cutting Edge | Fine Science tools | 15024-10 | |
| Student Halsey Needle Holder | Fine Science tools | 91201-13 | 3 needed: 2 for holding skin retractors and 1 for suturing the skin |
| Sucrose | Thermo Scientific | A15583.36 | For model validation |
| Universal Camera Ceiling Mount | Med Associates, Inc | ENV-598 | For model validation |
| water bath | VWR | 89032-226 | For model validation |
References
- Tuo, Q. Z., Zou, J. J., Lei, P. Rodent models of vascular cognitive impairment. J Mol Neurosci. 71 (5), 1-12 (2021).
- Zhou, Z., et al. Deeper cerebral hypoperfusion leads to spatial cognitive impairment in mice. Stroke Vasc Neurol. 7 (6), 527-533 (2022).
- Shibata, M., Ohtani, R., Ihara, M., Tomimoto, H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 35 (11), 2598-2603 (2004).
- Hattori, Y., et al. A novel mouse model of subcortical infarcts with dementia. J Neurosci. 35 (9), 3915-3928 (2015).
- Janson, J., et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 53 (2), 474-481 (2004).
- Herzig, M. C., et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 7 (9), 954-960 (2004).
- Miao, J., et al. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol. 167 (2), 505-515 (2005).
- Moechars, D., et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 274 (10), 6483-6492 (1999).
- Wallays, G., et al. Notch3 Arg170Cys knock-in mice display pathologic and clinical features of the neurovascular disorder cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Arterioscler Thromb Vasc Biol. 31 (12), 2881-2888 (2011).
- Cognat, E., Cleophax, S., Domenga-Denier, V., Joutel, A. Early white matter changes in CADASIL: evidence of segmental intramyelinic oedema in a pre-clinical mouse model. Acta Neuropathol Commun. 2, 49 (2014).
- Weng, Z., et al. A novel needle mouse model of vascular cognitive impairment and dementia. J Neurosci. 43 (44), 7351-7360 (2023).
- Biesbroek, J. M., Biessels, G. J. Diagnosing vascular cognitive impairment: Current challenges and future perspectives. Int J Stroke. 18 (1), 36-43 (2023).
- Winklewski, P. J., et al. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know. Front Neurol. 9, 92 (2018).
- Jing, Z., et al. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J Cereb Blood Flow Metab. 34 (10), 1613-1621 (2014).
- Yang, S. T., et al. Adult mouse venous hypertension model: common carotid artery to external jugular vein anastomosis. J Vis Exp. (95), e50472 (2015).
- Speetzen, L. J., Endres, M., Kunz, A. Bilateral common carotid artery occlusion as an adequate preconditioning stimulus to induce early ischemic tolerance to focal cerebral ischemia. J Vis Exp. (75), e4387 (2013).
- Lee, D., et al. A murine model of ischemic retinal injury induced by transient bilateral common carotid artery occlusion. J Vis Exp. (165), (2020).
- Schleimer, K., et al. Training a sophisticated microsurgical technique: interposition of external jugular vein graft in the common carotid artery in rats. J Vis Exp. (69), e4124 (2012).
- Speetzen, L. J., Endres, M., Kunz, A. Bilateral common carotid artery occlusion as an adequate preconditioning stimulus to induce early ischemic tolerance to focal cerebral ischemia. J Vis Exp. (75), e4387 (2013).
- Barone, F. C., Knudsen, D. J., Nelson, A. H., Feuerstein, G. Z., Willette, R. N. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 13 (4), 683-692 (1993).
- Wellons, J. C., et al. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 868 (1), 14-21 (2000).
- Cao, G., et al. In Vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 22 (13), 5423-5431 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved