Determining Rate Laws and the Order of Reaction
Genel Bakış
Source: Laboratory of Dr. Neal Abrams — SUNY College of Environmental Science and Forestry
All chemical reactions have a specific rate defining the progress of reactants going to products. This rate can be influenced by temperature, concentration, and the physical properties of the reactants. The rate also includes the intermediates and transition states that are formed but are neither the reactant nor the product. The rate law defines the role of each reactant in a reaction and can be used to mathematically model the time required for a reaction to proceed. The general form of a rate equation is shown below:
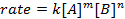
where A and B are concentrations of different molecular species, m and n are reaction orders, and k is the rate constant. The rate of nearly every reaction changes over time as reactants are depleted, making effective collisions less likely to occur. The rate constant, however, is fixed for any single reaction at a given temperature. The reaction order illustrates the number of molecular species involved in a reaction. It is very important to know the rate law, including rate constant and reaction order, which can only be determined experimentally. In this experiment, we will explore one method for determining the rate law and use it to understand the progress of a chemical reaction.
Prosedür
1. Preparing H2O2 Dilutions
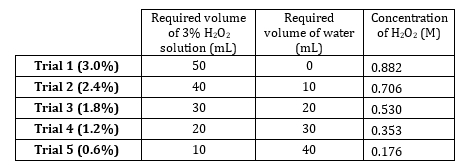
- Stock 3% hydrogen peroxide has a concentration of 0.882 M. Prepare 5 dilutions ranging from 0.882 M to 0.176 M (Table 1). Prepare these solutions volumetrically, but prepare them additively since the solute is very dilute and volumes of water are additive.
- Place the solutions in a constant temperature water bath or leave them on the bench top to equilibrate at room temperature. A temperature range of 20–25 °C (293–29
Sonuçlar
Oxygen Evolution Data and Initial Rates
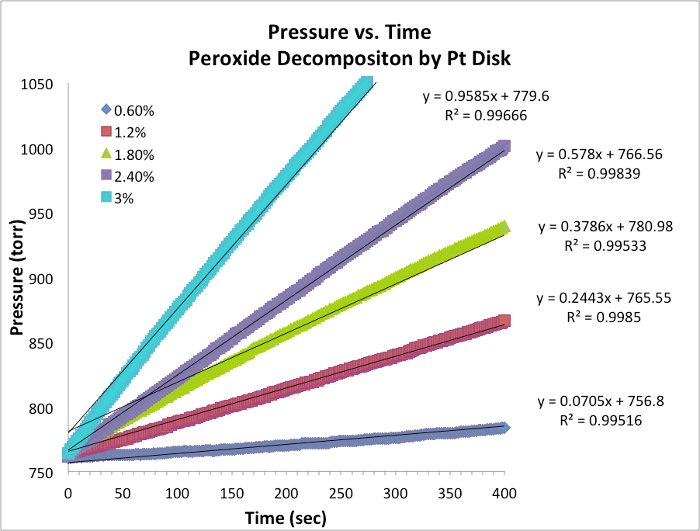
Figure 1. Pressure vs. time data for each trial at constant temperature. The slope is equivalent to the instantaneous rate of the reaction.
Reaction Order
- Data for five trials and graph with slope is tabulated below (Table
Başvuru ve Özet
While determining rate law variables can be involved mathematically, the methods are actually quite straightforward. As long as the disappearance of a reactant or appearance of a product can be measured, rate plots can used to calculate the rate constant. An extension of this method is frequently used to determine the activation energy of a reaction, Ea, by measuring the rate and calculating the rate constant at a variety of temperatures. This method involves using the Arrhenius equation, k = Ae(
Referanslar
- Method adapted from Vetter, T. A., Colombo, D. P. Jr. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide, J. Chem. Ed. 80 (7), 788-798 (2003).
- David R. Lide, ed. CRC Handbook of Chemistry and Physics. Boca Raton, Florida: CRC Press (2005).
Atla...
Bu koleksiyondaki videolar:

Now Playing
Determining Rate Laws and the Order of Reaction
General Chemistry
196.2K Görüntüleme Sayısı

Common Lab Glassware and Uses
General Chemistry
657.4K Görüntüleme Sayısı

Solutions and Concentrations
General Chemistry
274.7K Görüntüleme Sayısı

Determining the Density of a Solid and Liquid
General Chemistry
556.5K Görüntüleme Sayısı

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.7K Görüntüleme Sayısı

Determining the Empirical Formula
General Chemistry
183.0K Görüntüleme Sayısı

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.5K Görüntüleme Sayısı

Using a pH Meter
General Chemistry
346.6K Görüntüleme Sayısı

Introduction to Titration
General Chemistry
425.1K Görüntüleme Sayısı

Ideal Gas Law
General Chemistry
78.6K Görüntüleme Sayısı

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.6K Görüntüleme Sayısı

Le Châtelier's Principle
General Chemistry
265.7K Görüntüleme Sayısı

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.7K Görüntüleme Sayısı

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.5K Görüntüleme Sayısı

Coordination Chemistry Complexes
General Chemistry
91.6K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır
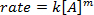 . Taking the natural logarithm (ln) of the equation produces a linear equation
. Taking the natural logarithm (ln) of the equation produces a linear equation 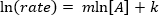 , where m, the slope, is the order of the reaction.
, where m, the slope, is the order of the reaction.