率の法律および反作用の順序を決定します。
概要
ソース: 博士ニール エイブラムスの研究室-環境科学および林業のニューヨーク州立大学
すべての化学反応は、反応しようとして製品の進行状況の定義特定率を持っています。この率は、温度、濃度、反応の物理プロパティによって影響があります。料金は、中間体や遷移状態が形成されるが、反応も製品にも含まれます。率法は反応の各反応の役割を定義し、続行する反応に必要な時間を数学的にモデル化するために使用できます。レート方程式の一般的な形は以下のとおりです。
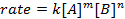
AとBが異なる分子種、 mとnの濃度を反応注文、 kは速度定数。ほぼすべての反応の速度は反応物質が枯渇している、有効な衝突が発生する可能性が低くを作るよう経年変化します。ただし、速度定数は、特定の温度における単一の反作用のため固定されています。反応次数は、反応に関与する分子種の数を示しています。率法、実験的に決定することができますのみ速度定数と反応順序などを知ることが非常に重要です。この実験では、探索率の法律を決定する 1 つの方法、化学反応の進行状況を理解するために使用します。
手順
1. 準備 H2O2希釈
- 株式 3% 過酸化水素 0.176 M (表 1) 0.882 M に至る 0.882 M. 準備 5 希釈の濃度があります。これらのソリューションを用いて、準備が、溶質は非常に希薄であり大量の水が相加的準備添加物。
- 恒温水槽にソリューションを配置または室温で平衡にベンチの上に置いておく。温度範囲は 20-25 ° C (293-298 K) はこの反応に適しています。
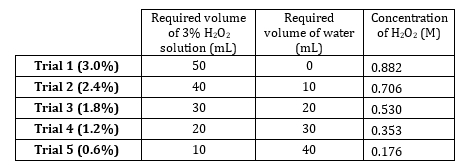
表 1。H2O2ソリューションを使用します。
2. 反応容器の準備
- 反応容器の体積を特定、上部に
結果
申請書と概要
参考文献
- Method adapted from Vetter, T. A., Colombo, D. P. Jr. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide, J. Chem. Ed. 80 (7), 788-798 (2003).
- David R. Lide, ed. CRC Handbook of Chemistry and Physics. Boca Raton, Florida: CRC Press (2005).
スキップ先...
このコレクションのビデオ:

Now Playing
率の法律および反作用の順序を決定します。
General Chemistry
196.1K 閲覧数

共通の実験室ガラス製品と用途
General Chemistry
656.3K 閲覧数

・濃度
General Chemistry
274.3K 閲覧数

固体と液体の密度を決定します。
General Chemistry
556.2K 閲覧数

水溶液の質量パーセントの組成を決定します。
General Chemistry
383.5K 閲覧数

経験式を決定します。
General Chemistry
181.9K 閲覧数

イオン性化合物の溶解度ルールの決定
General Chemistry
141.4K 閲覧数

PH メーターを使用してください。
General Chemistry
346.1K 閲覧数

滴定の概要
General Chemistry
424.6K 閲覧数

理想気体法律
General Chemistry
78.5K 閲覧数

平衡定数の吸光光度定量
General Chemistry
158.5K 閲覧数

ル Châtelier の原理
General Chemistry
265.4K 閲覧数

未知の化合物を決定するための凝固点降下
General Chemistry
160.7K 閲覧数

エンタルピーの示差走査熱量測定の変更を使用してください。
General Chemistry
44.5K 閲覧数

錯体化学
General Chemistry
91.5K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved
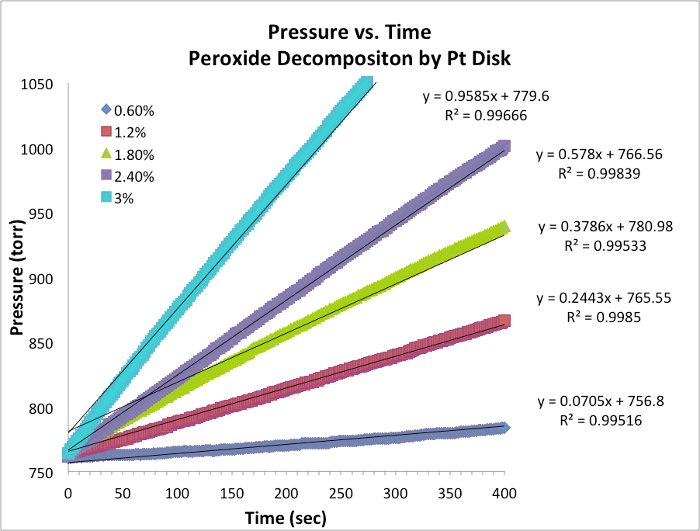
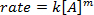 。線形方程式を生成する式の自然対数 (ln) を取って
。線形方程式を生成する式の自然対数 (ln) を取って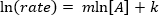 、 m斜面は反作用の順序。
、 m斜面は反作用の順序。