금리법 및 대응 순서 결정
Overview
출처: 닐 에이브람스 박사 연구소 — SUNY 환경과학 및 임업 대학
모든 화학 반응은 제품에 가는 반응의 진행을 정의하는 특정 속도를 가지고 있습니다. 이 속도는 반응제의 온도, 농도 및 물리적 특성에 의해 영향을 받을 수 있습니다. 속도는 또한 형성되는 중간 및 전이 상태를 포함하지만 반응제도 제품도 아니다. 속도 법은 반응에서 각 반응의 역할을 정의하고 반응진행에 필요한 시간을 수학적으로 모델링하는 데 사용할 수 있습니다. 금리 방정식의 일반적인 형태는 다음과 같습니다.
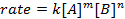
여기서 A와 B는 다른 분자 종의 농도이고, m와 n은 반응 순서이고, k는 일정한 비율이다. 반응제가 고갈됨에 따라 거의 모든 반응의 속도가 시간이 지남에 따라 변경되므로 효과적인 충돌이 발생할 가능성이 줄어듭니다. 그러나 속도 상수는 주어진 온도에서 단일 반응에 대해 고정됩니다. 반응 순서는 반응에 관여하는 분자 종의 수를 보여줍니다. 실험적으로만 결정할 수 있는 일정한 비율 및 반응 순서를 포함한 요금법을 아는 것이 매우 중요합니다. 이 실험에서는 속도법칙을 결정하는 한 가지 방법을 모색하고 이를 사용하여 화학 반응의 진행 상황을 이해할 것입니다.
Procedure
1. H2O2 희석제 준비
- 과산화수소 3%의 농도는 0.882M. 0.882M에서 0.176M(표1)에이르는 5희석을 준비한다. 이러한 솔루션을 체적으로 준비하지만, 솔루트가 매우 희석되고 물량이 첨가제이기 때문에 첨가제로 준비하십시오.
- 솔루션을 일정한 온도 수조에 놓거나 벤치 탑에 두어 실온에서 평형화하십시오. 20-25°C(293-298 K)의 온도 범위는 이 반응에 좋다.
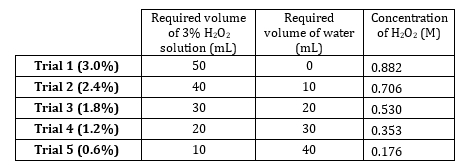
표 1. H2O2 솔루션 사용.
2. 반응 용기 준비
- 반응 용기의 부피를
Results
Application and Summary
속도 법 변수를 수학적으로 관련시킬 수 있지만 메서드는 실제로 매우 간단합니다. 제품의 반응제 나 외관의 실종을 측정할 수 있는 한, 속도 플롯을 사용하여 일정한 비율을 계산할 수 있습니다. 이 방법의 확장은 반응의 활성화 에너지를 결정하는 데 자주 사용된다,Ea,속도를 측정하고 다양한 온도에서 일정 속도를 계산하여. 이 방법은 Arrhenius 방정식, k = Ae(-Ea / RT)를사용?...
References
- Method adapted from Vetter, T. A., Colombo, D. P. Jr. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide, J. Chem. Ed. 80 (7), 788-798 (2003).
- David R. Lide, ed. CRC Handbook of Chemistry and Physics. Boca Raton, Florida: CRC Press (2005).
건너뛰기...
이 컬렉션의 비디오:

Now Playing
금리법 및 대응 순서 결정
General Chemistry
196.2K Views

일반적인 실험실 유리 제품 및 사용
General Chemistry
657.4K Views

솔루션 및 농도
General Chemistry
274.7K Views

고체 및 액체밀도 결정
General Chemistry
556.5K Views

수성 솔루션에서 질량 백분율 구성 결정
General Chemistry
383.7K Views

경험적 공식 결정
General Chemistry
183.0K Views

이온 화합물의 용해도 규칙 결정
General Chemistry
141.5K Views

pH 미터 사용
General Chemistry
346.6K Views

적정 소개
General Chemistry
425.1K Views

이상적인 가스 법
General Chemistry
78.6K Views

평형 상수의 분광측정 결정
General Chemistry
158.6K Views

르 샤텔리에의 원리
General Chemistry
265.7K Views

알 수 없는 화합물을 결정 하는 동결 포인트 우울증
General Chemistry
160.7K Views

차동 스캐닝 열량측정을 사용하여 엔탈피의 변화를 측정합니다.
General Chemistry
44.5K Views

조정 화학 단지
General Chemistry
91.6K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유
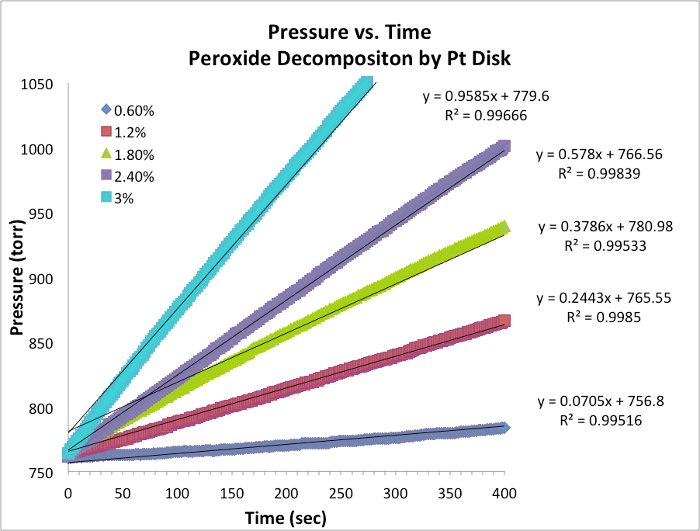
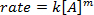 . 방정식의 천연 logarithm (ln)을 복용하면 선형
. 방정식의 천연 logarithm (ln)을 복용하면 선형 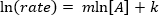 방정식을 생성하며, 여기서 m, 경사는 반응의 순서입니다.
방정식을 생성하며, 여기서 m, 경사는 반응의 순서입니다.