Determinación de las leyes de la velocidad y el orden de la reacción
Visión general
Fuente: Laboratorio de Dr. Neal Abrams — Universidad de SUNY de la ciencia ambiental y silvicultura
Todas las reacciones químicas tienen una tasa específica de definir el progreso de reactantes a productos. Esta tasa puede ser influenciada por la temperatura, concentración y las propiedades físicas de los reactivos. La tarifa incluye también los productos intermedios y Estados de transición que se forman son el reactivo ni producto. La ley tasa define el papel de cada reactivo en una reacción y puede utilizarse para modelar matemáticamente el tiempo necesario para que una reacción proceder. A continuación se muestra la forma general de una ecuación del tipo:
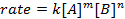
donde A y B son las concentraciones de diferentes especies moleculares, m y n son órdenes de reacción, y k es la constante de velocidad. La velocidad de cada reacción cambia con el tiempo como se agotan los reactivos, hacer colisiones eficaces menos probables que ocurra. La constante de velocidad, sin embargo, es fijo para cualquier sola reacción a una temperatura determinada. El orden de reacción ilustra el número de especies moleculares involucradas en una reacción. Es muy importante conocer la ley de la tarifa, incluyendo orden de tasa constante y reacción, lo que sólo puede ser determinado experimentalmente. En este experimento, exploraremos un método para la determinación de la ley de tasa y utilizarlo para comprender el progreso de una reacción química.
Procedimiento
1. preparación de H2O2 diluciones
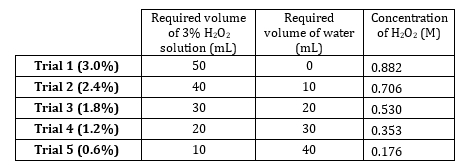
- Stock 3% peróxido de hidrógeno tiene una concentración de las diluciones de preparar 5 0,882 M. desde M 0,882 0,176 m (tabla 1). Preparar estas soluciones volumétricamente, pero prepararlos aditiva ya que el soluto es muy diluido y volúmenes de agua son aditivos.
- Colocar las soluciones en un baño de temperatura constante o dejarlas en la mesa se equilibren a temperatura ambiente. Una gama de temperaturas de 20 – 25 ° C (293 – 2
Resultados
Datos de evolución de oxígeno y tasas iniciales
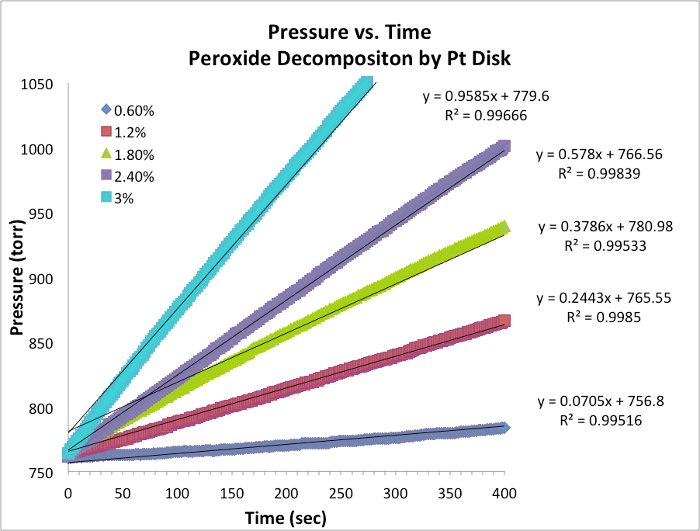
Figura 1. Presión vs tiempo datos para cada ensayo a temperatura constante. La pendiente es equivalente a la tasa instantánea de la reacción.
Orden de reacción
- Datos de cinco ensayos y gráfico con pendiente se tabulan a ...
Aplicación y resumen
Aunque determinar tasa ley variables puede estar implicado matemáticamente, los métodos son en realidad bastante sencillos. Como la desaparición de un reactivo o apariencia de un producto puede ser medida, tasa parcelas pueden utilizar para calcular la constante de velocidad. Una extensión de este método se utiliza con frecuencia para determinar la energía de activación de una reacción, Euna, por la tasa de medir y calcular la constante de velocidad en una variedad de temperaturas. Este método implica...
Referencias
- Method adapted from Vetter, T. A., Colombo, D. P. Jr. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide, J. Chem. Ed. 80 (7), 788-798 (2003).
- David R. Lide, ed. CRC Handbook of Chemistry and Physics. Boca Raton, Florida: CRC Press (2005).
Saltar a...
Vídeos de esta colección:

Now Playing
Determinación de las leyes de la velocidad y el orden de la reacción
General Chemistry
196.2K Vistas

Cristalería de laboratorio y usos comunes
General Chemistry
657.4K Vistas

Soluciones y concentraciones
General Chemistry
274.7K Vistas

Determinación de la densidad de un sólido y líquido
General Chemistry
556.5K Vistas

Determinación de la composición porcentual en masa de una solución acuosa
General Chemistry
383.7K Vistas

Determinación de la fórmula empírica
General Chemistry
183.0K Vistas

Determinación de las reglas de solubilidad de compuestos iónicos
General Chemistry
141.5K Vistas

Uso del medidor de pH
General Chemistry
346.6K Vistas

Introducción a la titulación
General Chemistry
425.1K Vistas

Ley del Gas ideal
General Chemistry
78.6K Vistas

Determinación espectrofotométrica de la constante de un equilibrio
General Chemistry
158.6K Vistas

Principio de le Châtelier
General Chemistry
265.7K Vistas

Depresión del punto de congelación para determinar un compuesto desconocido
General Chemistry
160.7K Vistas

Uso de la calorimetría diferencial para medir los cambios en la entalpía
General Chemistry
44.5K Vistas

Complejos de coordinación
General Chemistry
91.6K Vistas
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados
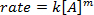 . Tomando el logaritmo natural (ln) de la ecuación produce una ecuación lineal
. Tomando el logaritmo natural (ln) de la ecuación produce una ecuación lineal 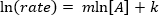 , donde m, la pendiente, es el orden de la reacción.
, donde m, la pendiente, es el orden de la reacción.