Case Report
Novel and Innovative Hybrid Technique for Type A Aortic Dissection
In This Article
Summary
The protocol describes ascending aortic replacement combined with endovascular coverage of the entire aortic arch with a fenestrated stent graft in a patient with acute type A aortic dissection in the absence of a tear in the aortic arch.
Abstract
Acute Stanford type A aortic dissection (TAAD) is a surgical emergency characterized by a high mortality rate and numerous complications. In the treatment of TAAD, the timing of surgery and the choice of surgical procedure are of paramount importance. Open total aortic arch reconstruction remains the gold standard for aortic arch surgery and is one of the most challenging procedures. However, this approach is invasive, relatively lengthy, and associated with substantial bleeding, which necessitates high levels of operator skill and carries the risk of multiple complications, particularly neurological ones. This report describes a novel hybrid procedure named Open Ascending Aorta Replacement Combined with Fenestrated Total Aortic Arch Stenting. A case was selected in which the lesion did not involve the aortic arch, at least on the greater curvature side of the arch. Ascending aortic replacement was performed, followed by arch intervention with self-modified stent grafts to preserve the aortic arch native branches. This approach allows for a rapid simplification of the procedure, avoids deep hypothermia or circulatory arrest associated with conventional open surgery, and mitigates neurological complications.
Introduction
Aortic dissection is a rare cardiovascular emergency associated with a high mortality rate; however, its incidence has increased in recent years while the age of onset has decreased, particularly for Stanford type A aortic dissection (TAAD)1,2. Aortic replacement remains the most common procedure utilized for TAAD3. Numerous postoperative complications are encountered, and mortality rates are elevated due to significant trauma and prolonged hypothermic circulatory arrest4,5.
The development of thoracic endovascular aortic repair (TEVAR) has led to the emergence of hybrid surgery6,7,8, making the procedure minimally invasive and less complex. Although strict indications exist, reduced blood loss, a shorter operative time, and the absence of deep hypothermic arrest mitigate the high risk of postoperative complications.
The hybrid surgery aims to shorten the time to functional recovery. The ascending portion of the aorta was replaced, regardless of whether the root was managed. The arch was fenestrated with stent grafts (SGs), and a stent covered the descending portion to enlarge the true lumen. This hybrid technique results in shorter operative time, decreased blood loss, and the risk of postoperative neurologic events and significant complications are comparable to or lower than those associated with open replacement. The surgical steps are simplified through reduced management of the three branches of the arch, compared to other previous hybrid surgeries9. Previous studies have demonstrated that hybrid surgery is characterized by reduced trauma and expedited recovery. It is acknowledged that numerous variations may exist at nearly every step of the procedure10,11.
This study presents an approach to hybrid surgery that incorporates TEVAR. Accurate identification and careful alignment, particularly of the three branches of the supra-arch, are critical. This case involves a 55-year-old male who presented with severe chest pain. Computed tomographic angiography (CTA) suggested TAAD without rupturing the arch. The patient consented to undergo hybrid surgery, followed by ascending aortic replacement and total arch using self-modified fenestrated SGs implantation (Figure 1), and was ultimately discharged from the hospital.
CASE PRESENTATION:
A 55-year-old male patient presented with chest tightness and pain that had started 11 h ago without any apparent trigger. He had a 3-year history of hypertension, with a maximum blood pressure of 150/100 mmHg, and was not taking any medication to control his blood pressure. He also had a 20-year history of gout, with no history of hyperlipidemia, diabetes mellitus, hepatitis B, or tuberculosis. He denied previous surgery, blood transfusions, drug or food allergies and reported no significant family history. On admission, the patient was alert and oriented and was receiving oxygen through a nasal cannula. Cardiac monitoring revealed a heart rate of 68 beats per min, oxygen saturation of 100%, respiratory rate of 16 breaths per min, and blood pressures of 126/83 mmHg in the left upper extremity, 139/79 mmHg in the right upper extremity, 135/80 mmHg in the left lower extremity, and 150/84 mmHg in the right lower extremity. The skin temperature of the upper limbs was cool, more so on the right side. Both pupils were equal in size, round, and approximately 3 mm in diameter, and were reactive to light. Breath sounds from both lungs were clear on auscultation, with no dry or wet rales. Heart sounds were normal, and no pathological murmurs were heard in any of the valvular auscultation sites. The abdomen was soft without tenderness or rebound pain. The liver and spleen were not palpable below the ribcage. The limbs showed normal muscle strength, and no oedema was noted in the lower limbs. Dorsal pedal pulses were palpable, and no pathological signs were elicited.
Diagnosis, Assessment, and Plan:
After the patient was admitted, appropriate tests and investigations were carried out. Cardiac echocardiography revealed the following diagnoses: 1. left ventricular hypertrophy, 2. proximal dilatation of the ascending aorta. The aorta was found to be abnormal, and further CTA examination confirmed the diagnosis of aortic dissection (Stanford A type) without rupture of the ascending aorta or arch. The dissection involved the superior mesenteric artery, the bilateral common iliac arteries, and the right external iliac artery. The right renal artery was supplied by the pseudocavity, and bilateral pleural effusions and inadequate expansion of the lower lung lobes were noted. Symptomatic treatment, including blood pressure and heart rate control and analgesia, was administered. The patient's diagnosis was confirmed, a head and abdominal examination was performed, contraindications to surgery were excluded, and the patient's family was provided with detailed information to facilitate preoperative preparation.
Protocol
Written informed consent was obtained from the patient for the procedure, and agreement was given to undergo ascending aortic replacement with the Fenestrated SGs. This study was conducted in compliance with all institutional, national, and international human welfare guidelines12 and received approval from the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Institutional Review Board document number TJ-IRB20220124). Written informed consent from the patients was obtained for the publication of the manuscript and any accompanying images.
1. Preoperative CTA evaluation
- The fenestration was designed according to the characteristics of the lesion, the precise measurement before the operation, and the configuration of the SG (Figure 2).
- The diameters of the aorta and the three major branching arteries were measured using CTA images, employing either the circular diameter or the elliptical mean diameter of the arteries. Measurements were taken at multiple anatomical landmarks, with particular attention to the aortic arch and its branches, which are crucial for the planning of hybrid surgery for TAAD.
- To obtain accurate measurements, a centerline was first defined along the length of the aorta and its branches. For each artery, the diameter was measured at the perpendicular cross-sectional plane that intersected the centerline at the chosen measurement site to avoid artifacts or vessel wall irregularities that might influence the accuracy of the measurements.
- The aorta was measured at key points, including the ascending aorta, the aortic arch (distal to the origin of the brachiocephalic artery), and the descending thoracic aorta. Similarly, the diameters of the major branches (brachiocephalic, left common carotid, and left subclavian arteries) were measured at the origin and distal points along the arch, ensuring consistency in measurement technique. The measurements were obtained using a calibrated software tool capable of generating precise cross-sectional views and enabling reliable diameter assessment.
- The angle of the aortic arch is the angle between the line between the proximal and distal ends of the aortic arch and the horizontal plane. Angle of arch was determined with the patient in the supine position. This angle determines the projection angle of the bulb when the aortic arch is deployed. It is usually left anterior oblique 30°-60°, with an average of 45°.
2. Ascending aortic replacement
- The appropriate artificial vascular graft (AVG) was selected based on the natural vessel diameter, as measured by CTA.
- The condition of the aortic sinus section, the intima of the aortic root, the coronary artery ostium, and the aortic valve leaflet structures were evaluated to determine whether the more proximal segment should be treated.
- In this case, the aortic valve was involved in the lesion, including involvement of the aortic annulus and regurgitation of the valve. After administration of heparin to achieve adequate anticoagulation, extracorporeal circulation (ECC) was established through the axillary and femoral arteries and the patient was not subjected to deep hypothermic cardiac arrest. ECC was assisted in order to safely perform the surgical intervention.
- The ascending aorta was then excised, and a tailored artificial graft (30 mm), selected based on the preoperative CTA measurements, was sutured in place to replace the diseased aortic segment. Concurrently, the aortic valve was replaced with a mechanical valve prosthesis to address valve dysfunction and ensure long-term hemodynamic stability.
- Throughout the procedure, the integrity of the coronary artery ostium was preserved by avoiding this zone for surgical operations and the aortic root structures were carefully inspected to ensure proper alignment of the graft and valve. The procedure aimed to achieve both immediate and long-term success in restoring aortic integrity and function.
3. SGs fenestration
- Following the completion of ascending aortic replacement, the chest was deliberately left open to facilitate subsequent evaluation of the distal anastomosis. To ensure precise identification of the anastomotic site for later assessment, Kelly clamps or titanium clips were placed at the location of the distal anastomosis. These markers serve as clear anatomical reference points for imaging and subsequent surgical interventions.
- After the markers were placed, digital subtraction angiography (DSA)13 was performed to assess the integrity and patency of the newly constructed aortic segment (Figure 3). The DSA imaging technique was employed to obtain high-resolution, real-time images of the distal anastomosis, allowing for precise evaluation of the blood flow dynamics and the absence of any complications such as stenosis, leakage, or malposition. The digital subtraction process enhances the visibility of the vascular structures by eliminating background tissue and highlighting the contrast-filled lumen.
- The position and length of the SG openings were determined based on intraoperative imaging and preoperative CTA data. In this case, the location of the descending tear was near the left subclavian artery.
- To avoid endoleak and incomplete lesion management, in situ windowing was planned for the left subclavian artery to implant the Viabahn, and in vitro fenestration was planned for the remaining two arch branches.
- First, the anterior end of the stent was covered, and it exceeded the distal aortic anastomosis by 10-15 mm. The length of SGs' window was defined by the proximal end of the brachiocephalic trunk ostium and the distal end of the left common carotid artery ostium. The width of the window was primarily determined by the diameter of the vessels in the arch and their relative position to each other.
- To accurately position the SGs' windows with the corresponding branches of the aortic arch, fixed sutures of radiopaque materials such as stainless-steel wire or thin metal plates were used at both ends of the SG window. These materials are chosen for their radiopacity, which allows clear visualization under fluoroscopic guidance during the procedure.
- Suturing was performed with careful placement of either interrupted or continuous sutures through the SGs at the edges of the window. The metal wire or sheet was attached to the stent at these points to ensure stable positioning and precise alignment of the modified window with the arch vessels, particularly at the level of the brachiocephalic trunk and left common carotid artery, to minimize the risk of complications such as restenosis or misalignment of the branch ostium.
- If the branches were farther apart on the bow, separate windows were used for each of the three branches, requiring accurate measurements from a 3D image.
4. SGs implantation
- Modified SGs were implanted through the femoral artery (Figure 4).
- The handle of SGs was rotated, and the SG was released before slowly unsheathing it and opening the bracket to the starting position of the bracket overlay. The side of the SG corresponding to the white dot on the handle is identified as the opening side, and the opening side marker was confirmed.
- The anterior and posterior positions of the opening window may also be marked using the corresponding SG segments. The position of the aortic arch and the branch within the arch was repeatedly determined by marking the aortic arch and two branches with screen markers and corresponding markers on the bone.
- A guidewire was advanced through the sheath into the left subclavian artery, with real-time fluoroscopy guiding the procedure to ensure accurate catheter placement at the site corresponding to the previously deployed SGs, after which a balloon catheter was introduced through the sheath and advanced to the ostium of the subclavian artery.
- Balloon dilation was carefully performed at the site of the SGs to optimize stent expansion, improve apposition to the aortic wall, and restore flow in the dissected aorta. The balloon was inflated gradually, and the pressure was carefully monitored to avoid vessel injury while ensuring adequate stent expansion. After successful balloon dilatation, a Viabahn-covered stent graft was deployed across the site to minimize the risk of endoleak and secure the position of the SGs.
- Depending on the patient's needs, a second stent was implanted in the descending aorta to eliminate as much of the false lumen as possible.
5. Positioning of the guidewire
- In the TEVAR procedure for TAAD, the implantation of SGs requires meticulous guidewire handling to ensure precise stent deployment. Initially, a guidewire was selected and carefully shaped to match the aortic anatomy. To support the guidewire appropriately and prevent any complications during advancement, a branched or pretreated AVG was used.
- Once the guidewire was securely in place, the SG was advanced over the guidewire, ensuring smooth, controlled deployment at the target site using fluoroscopic guidance.
- In this case of hybrid surgery for TAAD, the implantation of the stent graft (SG) was complicated by a short replacement aortic graft (AVG) and the presence of a mechanical aortic valve, which impeded the passage of the guidewire through the valve. To overcome this challenge, a branched or pretreated AVG was utilized, which allowed the guidewire to be positioned above the aortic valve, avoiding direct interaction with the valve's mechanical components.
- The AVG was carefully advanced so that the guidewire protruded from the graft, anchored outside the lumen of the aortic valve. This provided a stable, secure pathway for advancing the guidewire through the aorta while preventing any potential damage to the valve or disruption of its function. The guidewire's position was carefully monitored using fluoroscopy to ensure its proper placement above the valve.
- Additionally, since the replacement AVG is short and the aortic valve has not been replaced, the guidewire was extended into the ventricle. The tip was pretreated to ensure it was curved, reducing the risk of heart damage. However, when the replacement AVG is of ideal length, the aortic valve does not affect the guidewire, and it was placed directly into the vessel.
6. Postoperative DSA
- Following the surgical procedure, DSA was performed to assess the outcome of the hybrid repair in the patient. DSA provided high-resolution imaging, allowing for detailed visualization of the entire aorta, including the three major branches of the aortic arch (brachiocephalic trunk, left common carotid artery, and left subclavian artery). This was crucial to ensure that the aortic arch was well patent, with no evidence of stenosis or any compromise to the flow of blood to the head, neck, and upper limbs.
- Additionally, DSA enables the assessment of the newly deployed SGs, confirming that they were properly positioned without kinks or displacements. The procedure was also critical in detecting any potential complications, such as internal leakage, stent migration, or endoleaks, which could compromise the repair and necessitate further intervention. Smooth, unobstructed blood flow across the aortic arch and into the branches was used as an indicator of a successful outcome, ensuring that the hybrid procedure has effectively restored normal hemodynamics.
- After the DSA confirms the absence of any leakage and the successful placement of the stent graft, the chest was then closed in layers. This involves the careful closure of the pericardium, followed by the reapproximation of the chest wall muscles, fascia, and skin, ensuring proper hemostasis and minimizing the risk of postoperative complications such as infection or wound dehiscence.
Results
The representative results of this case demonstrate the technical success and feasibility of the hybrid approach to TAAD. The operation was completed in a reasonable time frame of 6 h with a controlled blood loss of 500 mL, reflecting the minimally invasive nature of the hybrid approach compared to traditional open surgery. The patient's rapid recovery, waking up just 3 h post-operatively without any sensory or motor abnormalities, is an important indicator of the effectiveness of the procedure in maintaining neural and vascular integrity. The absence of complications such as neurological deficits and the fact that the patient did not require deep hypothermia during the procedure highlights the reduced physiological stress imposed by the hybrid technique.
In addition, post-operative CTA imaging (Figure 5), which showed no significant contrast leakage, no stent dislodgement, and smooth blood flow in the three branches of the aortic arch, supports the technical success of the stent placement and confirms the patency and stability of the repair. These imaging results are critical in demonstrating the efficacy of the hybrid procedure in repairing the TAAD and restoring normal blood flow to the head, neck, and upper extremities. The patient was discharged on postoperative day 11 with no major complications, further underscoring the favorable outcome and rapid recovery associated with this approach.
To analyze the outcome, it is important to assess both the immediate post-operative results, as seen in the imaging and recovery data, and the longer-term follow-up to evaluate the durability of the stent graft and the possibility of late complications such as endoleaks or restenosis. In addition, comparing this hybrid approach with traditional surgical techniques in terms of operative time, blood loss, and complication rates could provide valuable insights into the benefits of hybrid surgery in TAAD.
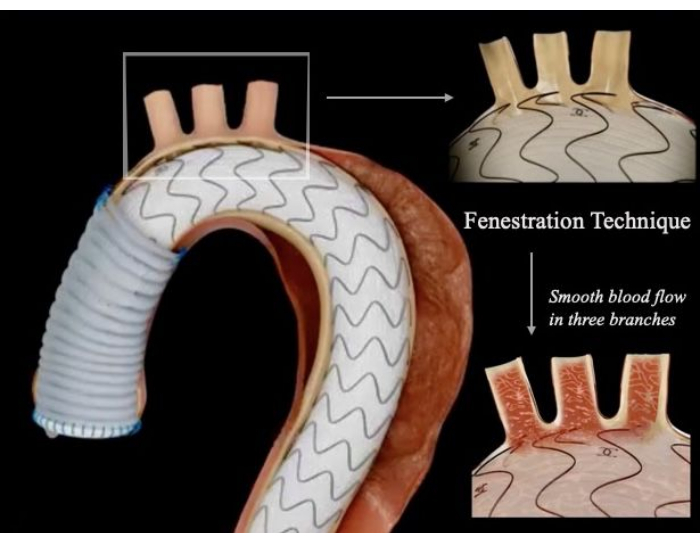
Figure 1: Schematic diagram of the hybrid technique ascending aortic replacement combined with a fenestrated stent graft. The fenestrated site was precisely aligned with the branches of the arch, allowing smooth blood flow to the head, neck, and upper extremities and complete removal of the aortic lesion without endoleak. Please click here to view a larger version of this figure.
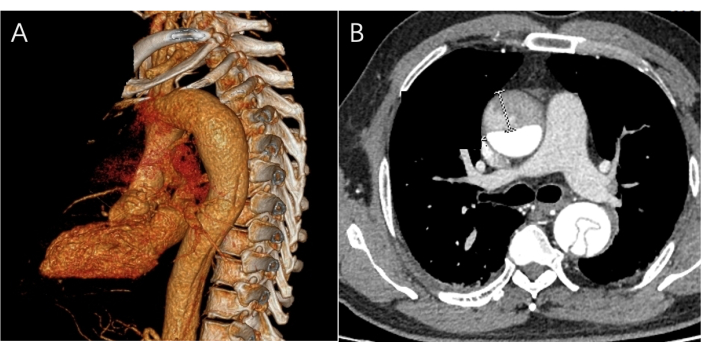
Figure 2: Images of preoperative CTA. (A) A three-dimensional CTA image of the site of the lesion is visible, but no tears are present in the aorta. (B) The image of the CTA transverse Plane shows the ascending and descending parts of the aorta presenting a double lumen with an intimal flap. Please click here to view a larger version of this figure.
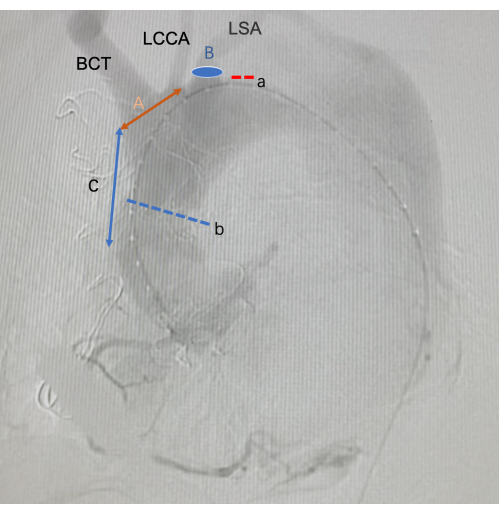
Figure 3: Images of intraoperative DSA. a is the tear of the aortic dissection. b is the distal anastomosis of an artificial vascular graft. A is the length of the in vitro fenestration. B is the position of the in-suit fenestration. C is the length of the starting position of the SG fenestration from the anterior end of the SG. Abbreviations: BCT =Brachiocephalic trunk; LCCA =Left Common carotid artery; LSA =Left Subclavian artery Please click here to view a larger version of this figure.
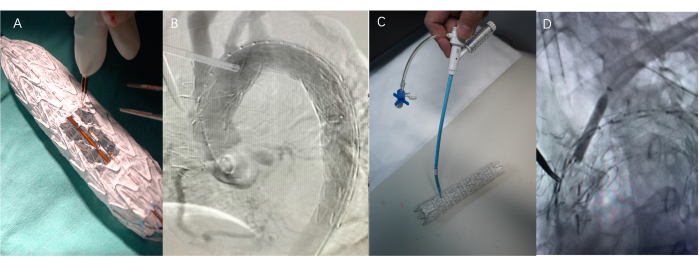
Figure 4: Surgical procedure. (A) Self-modification of stent grafts using a cautery pen or scalpel - Fenestrated surgery. The length of the window is the total length of the protrusions of the branches, and the width is the diameter of the branches. (B) The process of implanting a stent graft (SG). (C) Adjustable bending and piercing needles used in in-situ fenestration technology. This device can flexibly adjust the angle and position of the front end. (D) Use a balloon to dilate the puncture site to facilitate implantation of the Viabahn after the needle has passed through the covered stent. Please click here to view a larger version of this figure.
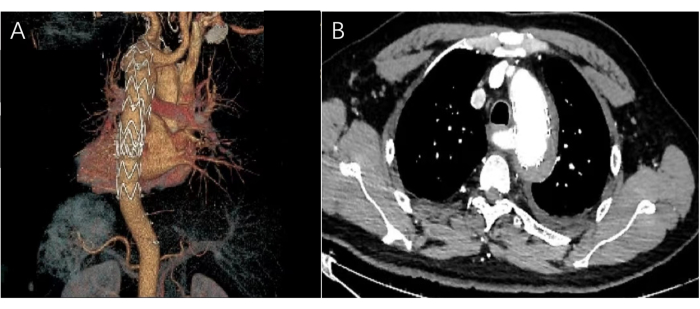
Figure 5: Images of postoperative CTA. (A) The post-operative three-dimensional CTA image shows that the aortic stent is in the aortic arch, and the left subclavian artery tear is completely closed. (B) The horizontal CTA image shows the shadow of the stent with no hematoma or contrast leakage. Please click here to view a larger version of this figure.
Discussion
This procedure is currently indicated for selected patients with quality aortic arches, such as those with 1) tears in the descending and/or ascending aorta where the aortic arch is sufficiently intact to allow the use of a blocking clamp, with no tears on the greater curvature side and no entrapment of the supra-arterial branches; 2) even if there are tears in the arch, they are confined to the lesser curvature side and the TEVAR procedure will isolate the tears, minimizing the risk of internal leakage. The key steps in the fenestration procedure are as follows: ascending aortic replacement, intra-operative DSA examination, fenestration of the SGs, implantation of modified SGs that cover and extend the anastomosis by 10-15 mm, localization and docking to the arch branch, and use of a super-stiff guidewire.
This procedure requires fewer anastomoses, reduces the number of surgical steps, is easy to perform, and avoids deep hypothermic circulatory arrest14. In TEVAR, the chest is not sutured, and only an adhesive membrane is used, primarily to avoid re-heparinization and re-administration of protamine, which increases the risk of thoracic bleeding and failure to detect bleeding in time. In addition, the adhesive membrane provides a support point if the stent has difficulty crossing the distal anastomosis. In the event of malposition, the problem can be addressed promptly, for example, by creating a bypass or performing a needle puncture of the membrane. In addition to the usual surgical complications, neurological complications should be closely monitored postoperatively15,16. Treatment of the arch can affect the blood supply to the head, neck, and upper extremities. Because of this risk, a DSA is performed at least postoperatively in the operating theatre to assess the blood supply to the arch branches. The motor and sensory function of the affected limb should be assessed as soon as possible when the patient is awake. The stability of the SGs may be compromised after open surgery on the overlying stent, and there is a risk of SG migration. This hybrid procedure requires a high level of skill from the cardiac surgeon, who must not only be proficient in open surgical techniques but also have advanced endovascular skills.
By ensuring that the anchorage zone is sufficiently long, the use of an overlay stent can minimize the number of procedural steps17. The design of the overlay stent can be tailored to the patient's lesion characteristics18. For example, if the three branches of the supra-aortic arch are widely spaced, double or triple windows can be selected to maintain stent stability. When the supra-aortic branches of the arch are involved, such as in the case of a clipped infraclavicular artery, in vitro fenestration combined with in situ fenestration can be used to implant a branch stent into the vessel, thereby reducing the risk of endoleak. This approach can help ensure stent stability, as demonstrated in this case.
Hybrid surgery is feasible, but future prospective studies are needed to validate this approach. The technique described, in combination with adequate CTA measurements and precise rupture localization, may offer a valuable alternative to both traditional open surgery and classic hybrid procedures. Further studies are needed to compare the short and long-term outcomes of this hybrid procedure with those of open surgery and classical hybrid surgery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgments.
Materials
| Name | Company | Catalog Number | Comments |
| Adjustable bend | Lifetech | 106938370117414.00 | The Lifetech Adjustable Bend is constructed from high-quality, biocompatible materials, ensuring both flexibility and durability. The outer layer is typically composed of a polyurethane or similar material that is resistant to kinking, while the inner components include a nickel-titanium alloy (nitinol), known for its superelastic properties, which allow the catheter to return to its original shape after bending to improve the navigability and adaptability of endovascular procedures in challenging vascular anatomies. |
| Artificial vascular graft | Terumo | 734006 | The artificial vascular graft used in this study is made from expanded polytetrafluoroethylene (ePTFE), a biocompatible synthetic material used in vascular surgeries. |
| Balloon catheter | Boston Scientific | H74939171060410 | The Boston Scientific B-Balloon Catheter is a highly advanced, precision-engineered device designed for use in percutaneous transluminal angioplasty (PTA) procedures, particularly in vascular interventions. Its key feature is the balloon catheter technology, which allows for the effective dilation of stenotic lesions in both peripheral and coronary arteries. |
| Guidewire | Cook Medical | G14544 | The Cook Guidewire is a high-performance medical device used to navigate and guide catheters, balloons, and other devices in interventional procedures. It is made from durable materials such as stainless steel and nickel-titanium alloy and is available in a range of sizes and designs tailored to specific clinical needs. The guidewire features a flexible, torqueable, and pushable structure that allows precise navigation through challenging anatomical pathways. |
| Mechanical valve | Medtronic | A7700 | The mechanical heart valve is a widely used prosthetic device designed for the replacement of damaged or diseased heart valves and is particularly suitable for younger patients who require a long-lasting solution for valve replacement, with a proven clinical track record of over 20 years of durability. |
| Pigtail catheter | Cook Medical | G11916 | The Cook Pigtail Catheter is constructed from radiopaque materials, allowing for clear visualization under fluoroscopy,and a versatile, reliable device that enhances the effectiveness of various diagnostic and therapeutic interventions. Its flexible, radiopaque design and pigtail shape provide stability and ease of navigation, making it a valuable tool for clinicians performing cardiac and vascular procedures. |
| Stent-graft | Lifetech | (01)06938370139126 | The Lifetech Stent-Graft is a highly effective and reliable solution for the endovascular treatment of a variety of vascular conditions, particularly aortic dissection.It has a discontinuous, non-radiopaque metal strip on the back.Its hybrid design, incorporating a self-expanding nitinol stent with a durable, biocompatible graft, provides both structural support and sealing, offering significant advantages over traditional open surgery in terms of patient recovery and procedural risk. |
| Stent-graft | Medtronic | VAMF3232C200TE | The Medtronic Stent-Graft represents a significant advancement in the field of endovascular surgery, offering a safe and effective alternative to open surgical repair for patients with complex vascular pathologies such as aortic dissection. The combination of a self-expanding nitinol stent with a durable, biocompatible graft provides optimal sealing and long-term durability. |
| Viabahn | Gore | VBHR080202W | The Gore Viabahn Stent-Graft is composed of a stainless steel or nitinol stent covered by a ePTFE (expanded polytetrafluoroethylene) graft.The Viabahn combines the mechanical support of a self-expanding stent with the sealing capabilities of a biocompatible graft, providing a durable, minimally invasive solution to treat complex vascular lesions. |
References
- Zhu, Y., et al. Type A aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J Am Coll Cardiol. 76 (14), 1703-1713 (2020).
- Kallenbach, K., et al. Treatment of the aortic root in acute aortic dissection type A: insights from the German registry for acute aortic dissection type A. Eur J Cardiothorac Surg. , ezac261 (2022).
- Hagan, P. G., et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 283 (7), 897-903 (2000).
- Mousavizadeh, M., et al. Hypothermic circulatory arrest time affects neurological outcomes of frozen elephant trunk for acute type A aortic dissection: A systematic review and meta-analysis. J Card Surg. 36 (9), 3337-3351 (2021).
- Pupovac, S. S., et al. Moderate versus deep hypothermia in type A acute aortic dissection repair: Insights from the international registry of acute aortic dissection. Ann Thorac Surg. 112 (6), 1893-1899 (2021).
- National Society of Vascular Surgery, China. Chinese expert consensus on hybrid technique on treating thoracic aortic pathologies involving the aortic arch. Chinese Circ J. 35 (2), 124-130 (2020).
- National Committee of Experts on Cardiovascular Diseases. The Chinese expert consensus on hybridization techniques for treatment of aortic diseases involving the arch. Chinese J Circ. 35 (2), 124-130 (2020).
- Zhang, L., et al. Hybrid and frozen elephant trunk for total arch replacement in DeBakey type I dissection. J Thorac Cardiovasc Surg. 158 (5), 1285-1292 (2019).
- Liu, X., et al. Hybrid total arch replacement via ministernotomy for Stanford type A aortic dissection. Front Cardiovasc Med. 10, 1231905 (2023).
- Liu, Y., et al. Early outcomes of hybrid type II arch repair versus total arch replacement with frozen elephant trunk in acute DeBakey type I aortic dissection: a propensity score-matched analysis. Interact Cardiovasc Thorac Surg. 31 (4), 565-572 (2020).
- Liu, S., et al. Midterm outcomes of one-stage hybrid aortic arch repair for stanford type A aortic dissection: A single center's experience. Semin Thorac Cardiovasc Surg. 35 (2), 311-321 (2023).
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 310 (20), 2191-2194 (2013).
- Dai, L., et al. Safety and effectiveness of the sutureless integrated stented graft prosthesis in an animal model. Heliyon. 10 (9), e30323 (2024).
- Sirota, D. A., et al. Hybrid technologies for reconstruction of proximal aortic dissection. Sovrem Tekhnologii Med. 15 (3), 42-51 (2023).
- Jensen, C. W., Chen, E. P. Management of brain malperfusion in acute type A aortic dissection. Asian Cardiovasc Thorac Ann. 30 (3), 364-370 (2022).
- Gaul, C., Dietrich, W., Erbguth, F. J. Neurological symptoms in aortic dissection: a challenge for neurologists. Cerebrovasc Dis. 26 (1), 1-8 (2008).
- Kuzniar, M. K., Wanhainen, A., Tegler, G., Mani, K. Endovascular treatment of chronic aortic dissection with fenestrated and branched stent grafts. J Vasc Surg. 73 (5), 1573-1582 (2021).
- Tenorio, E. R., et al. Fenestrated and branched aortic research Consortium investigators. Outcomes of endovascular repair of chronic postdissection compared with degenerative thoracoabdominal aortic aneurysms using fenestrated-branched stent grafts. J Vasc Surg. 72 (3), 822-836.e9 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved