Method Article
异位自体肾移植的猪模型:有步骤,分步协议
摘要
Porcine models of organ transplantation provide an important platform to study mechanisms of organ preservation. This article describes a heterotopic porcine renal autotransplantation model, which allows investigating new approaches to improve the outcome of transplantation using marginal kidney grafts.
摘要
Kidney transplantation is the treatment of choice for patients suffering from end-stage renal disease. It offers better life expectancy and higher quality of life when compared to dialysis. Although the last few decades have seen major improvements in patient outcomes following kidney transplantation, the increasing shortage of available organs represents a severe problem worldwide. To expand the donor pool, marginal kidney grafts recovered from extended criteria donors (ECD) or donated after circulatory death (DCD) are now accepted for transplantation. To further improve the postoperative outcome of these marginal grafts, research must focus on new therapeutic approaches such as alternative preservation techniques, immunomodulation, gene transfer, and stem cell administration.
Experimental studies in animal models are the final step before newly developed techniques can be translated into clinical practice. Porcine kidney transplantation is an excellent model of human transplantation and allows investigation of novel approaches. The major advantage of the porcine model is its anatomical and physiological similarity to the human body, which facilitates the rapid translation of new findings to clinical trials. This article offers a surgical step-by-step protocol for an autotransplantation model and highlights key factors to ensure experimental success. Adequate pre- and postoperative housing, attentive anesthesia, and consistent surgical techniques result in favorable postoperative outcomes. Resection of the contralateral native kidney provides the opportunity to assess post-transplant graft function. The placement of venous and urinary catheters and the use of metabolic cages allow further detailed evaluation. For long-term follow-up studies and investigation of alternative graft preservation techniques, autotransplantation models are superior to allotransplantation models, as they avoid the confounding bias posed by rejection and immunosuppressive medication.
引言
Kidney transplantation is the treatment of choice for patients with end-stage renal disease, due to associated lower rates of morbidity and mortality when compared to dialysis 1-3. Despite major improvements in patient outcomes following kidney transplantation, graft shortage still poses a severe challenge worldwide. The number of patients waiting for a kidney transplant by far exceeds the number of organs available 4-6. To increase the number of kidneys available for transplantation and to reduce patient waiting times, further sources of kidney grafts are needed.
Commonly, standard criteria donor (SCD) and extended criteria donor (ECD) kidney grafts from donation after brain death (DBD) as well as kidneys recovered from live donors (LDKT) are utilized. Since the 1990s, an increasing number of kidney grafts have been recovered in a donation after circulatory death (DCD) scenario, to further expand the donor pool 7,8. However, DCD and ECD kidney grafts demonstrate acceptable but decreased outcomes after transplantation, depending on different factors, such as donor age, warm and cold ischemia times, and the preservation technique used 9-11. Thus, additional research is required to improve the outcome of patients receiving marginal kidney grafts and to further increase the donor pool.
The porcine model of renal transplantation is well established and provides a clinical important scenario to investigate innovative approaches for the improvement of marginal kidney graft outcomes. In contrast to rodent and canine kidneys, which are unilobular, porcine and human kidneys are multilobular and are anatomically similar, particularly in regard to the arterial, venous, and urinary collecting systems 12,13. In addition, porcine and human kidneys demonstrate similarities in the pathophysiology of ischemia reperfusion injury (IRI), biochemistry, and immunological parameters 14. Thus, porcine renal transplantation is well-suited to investigate new organ preservation methods for marginal kidney grafts 15-17, model human IRI 18, study immunological pathways and allograft tolerance 19, provide surgical training 20-22, test new pharmacological therapies 23, implement new medical devices, and study new immunological mechanisms in xenotransplantation 24-26.
The renal porcine and human transplantation settings are not completely analogous. This article focuses on important technical details that will facilitate successful establishment of a renal autotransplantation model. Species-adapted pre- and postoperative housing, administration of anesthesia with close monitoring, and matched surgical techniques are described in the protocol and demonstrated in the video. Resection of the contralateral native kidney provides the opportunity to assess the function of the transplanted kidney. The placement of venous and urinary catheters and the use of metabolic cages allow more in-depth assessment. For studies aimed at investigating alternative graft preservation methods and mechanisms of IRI, autotransplantation models are superior to allotransplantation models, as they avoid the complications and confounding bias associated with rejection and use of immunosuppressive medications.
研究方案
所有动物人文关怀,我们按照加拿大议会关于动物保护的政策和指导方针进行了所有的研究。所有的程序都被下批准的大学健康网络机构动物护理委员会,动物使用的协议进行的。
注意:研究方案的示意性概述图1中。
图1.研究协议。 请点击此处查看该图的放大版本。
1.动物
- 在本协议中使用的男性约克夏猪(30公斤)。
2.移植肾检索
- 术前程序
- 家雄Yorkshir在至少一个星期的研究机构Ë猪适应新环境他们。使用肌肉注射的第三代头孢菌素,如头孢噻呋,3天,以减少与猪链球菌 和沙门氏菌感染的潜在风险。快猪进行麻醉,防止吸入诱导前至少6小时。
- 氯胺酮(20毫克/千克),阿托品(0.04毫克/千克),咪达唑仑(0.3毫克/千克)的肌肉注射引发猪麻醉。接着,从壳体设施到手术室(OR)运输的动物。
- 放置在猪上或桌子仰卧位。允许猪自发呼吸2升氧气的异氟醚的5%。暴露声带用喉镜,并用2%利多卡因局部溶液喷洒防止插管诱导喉痉挛。用6.5毫米管插管后,方框用3-5毫升的空气的压脉袋。
注:Capnometry确认正确的positio气管管的n个。 - 降低异氟烷气体至2.5%。呼吸机设置为14-16次/分钟,潮气量到10-15毫升/公斤体重。密切监测猪。心脏速率和氧饱和度是由脉搏血氧记录。确认通过降低心脏速率适当麻醉(低于150次/分)和血压(低于100毫米汞柱的收缩值),以及没有猪动作(无肌肉松弛剂的使用量)。
- 在无菌条件下,引入一个9.5大一单腔永久导管进入使用Seldinger技术27的颈内静脉。简单地说,用针穿刺静脉。引入导丝后,替换为剥离式导引针,接着置换的血管导管的导线。固定导管用3-0丝线或不可吸收缝线丝皮肤。
- 施用500mg的甲硝唑,1克头孢唑啉,和20mg泮托拉唑。广告部长200毫升用5%右旋糖(D5W)和每小时1芬太尼毫升柠檬酸静脉内整个手术乳酸林格氏溶液。对眼睛应用兽医眼药膏,以防止干燥时的麻醉下。
- 手术过程
- 以下无菌消毒和外科领域的覆盖范围,执行在长25厘米的正中切口。插入牵开。覆盖用毛巾大大小小肠子,并将它们放置到左侧为到右肾最佳接入。
- 自由输尿管和使用烧灼任何附着组织右肾本身。
- 解剖右肾静脉和动脉使用烧灼直到从下腔静脉和主动脉产地,分别是免费的。为了避免动脉血管痉挛,30〜65毫克罂粟碱政府应予以考虑。
- 经过完整的肾切除,领带(丝绸,3-0)和远端切断输尿管。 Prepar冰碗EA和无菌风琴袋。
- 首先,夹住肾动脉靠近主动脉和第二,夹住肾静脉接近使用血管钳腔静脉。接下来,切除移植肾立即导管插入肾动脉与肾动脉插管。用500毫升冰冷的组氨酸 - 色氨酸酮戊二酸(HTK)溶液冲洗掉的血液。存放在冰肾移植前。
- 在现场 ,关闭剩余的肾动脉用结扎(丝绸,2-0)和肾静脉用连续缝合(普理灵,6-0)。
- 检查出血的解剖区域后,关闭腹壁连续缝合(单丝,1),并以3-0丝线或不可吸收缝线丝皮肤..
- 术后程序
- 修正了一个缝合线(丝,3-0)和隧道它来猪的背静脉导管皮下,以防止不必要的操作。将猪后容易发生,Suture(丝,3-0)将导管稳固地在皮肤上。
- 断奶从呼吸机的猪,让它在拔管后其住房面积恢复。辖乳酸林格氏液静脉滴注,体积膨胀和管理镇痛0.3毫克丁丙诺啡。无人看管,直到它已经恢复了足够的意识,以保持胸骨斜卧不要让动物。
3.肾移植术
- 术前程序
- 麻醉使用丙泊酚的静脉注射(1-2毫克/公斤体重),然后在50-100毫克/小时的速度异丙酚连续输注的猪。如步骤2.1.3和2.1.4所述重新插管猪和异氟醚气体设定为3-4%。
- 辖1克头孢唑啉和20mg泮托拉唑静脉的手术过程中,使用相同的麻醉协议在2.1.4中描述。
- 以下无菌消毒,使4厘米的切口旁边的气管。剖析TIssue以暴露颈动脉。通过霍尔特镊子和丝绸领带(2-0)周围的动脉。使用Seldinger技术引进塑料导管连续测量整个手术的动脉压。可替代地,非侵入性的血压测量技术可被利用。
- 手术过程
- 无菌消毒后,通过切割皮肤和筋膜缝合的线圈重新打开腹腔,重新引入手术用牵引器具,以暴露腹腔,并重新定位肠到左侧,以允许对肾血管更好的访问。
- 移植保存肾移植结束到另一侧的肾下腔静脉和主动脉。因此,解剖腔静脉和主动脉超过5-8厘米用皮卡和烧灼髂分叉以上。如果可能的话,不打扰淋巴管;如果没有可能,用5-0普理灵缝线关闭它们。
- 在完成解剖后,检查FOř出血,并从容器中移除剩余的组织。确保腔静脉和主动脉与Satinsky钳的完整的夹紧是可行的。
- 接下来,切除对侧(左)肾。为此,定位肠向右;解剖输尿管,肾脏本身,肾静脉,并从组织附着肾动脉。扎输尿管和血管切除肾脏。检查出血。
- 重新定位肠向左露出下腹主动脉和腔静脉。注入肝素(100IU / kg体重),并等待至少2分钟。
- 静脉吻合:
- 使用Satinsky钳完全夹紧腔静脉,并相匹配的肾静脉的开口的大小,使用11刀片的狭缝切口。波特剪刀可用于进一步扩大狭缝。
- 包裹肾脏到含有无菌冰布后,从冰中取出,并将其放置到手术区域。使用两个双武装6-0普理灵缝线执行颅和尾部角落针。
- 近似肾,扎上一角,用6-0普理灵,从后墙进行连续缝合。在完成2/3后,使用领带的另一端的前端,完成缝合。追平了颅缝后,在尾角落扳平针。
- 定位在肾静脉的牛头犬夹,打开Satinsky夹。检查吻合出血。
- 动脉吻合:
- 使用Satinsky再次夹紧到完全夹紧主动脉。使用一个11叶片使狭缝切口,匹配肾动脉的开放。使用4.0毫米的圆形打孔,以确保干净的开放。
- 使用一个6-0 Prolene线缝合执行动脉吻合,开始于收件人侧。确保动脉内皮包含在每个缝合以防止清扫。同时,开始以10ml连续滴注norepinephrINE(16毫克/ 250毫升)稀释在500ml乳酸林格氏液和滴定以保持高于100毫米汞柱的收缩压。
- 动脉吻合完成之前注入维拉帕米动脉内和局部给药罂粟碱到容器,以防止血管痉挛的外部。
- 定位在肾动脉的牛头犬夹,打开Satinksy夹。检查吻合出血。
- 解开软布上的肾取出冰块。打开静脉斗牛犬钳第一,其次是动脉牛头犬夹。再灌注后,尿量应立即启动。
- 用布为保证移植的移植物的有利位置,并保持均匀的再灌注。
- 输尿管吻合术:
- 使用波特剪刀剪开从移植输尿管和收件人超过0.5厘米的纵向长度。
- 使用两个6.0聚酯,聚(对 - 二恶烷酮)缝合的侧到另一侧的URE或双侧吻合。在每一侧执行的角施蒂希,然后运行以连续方式第一后壁,随后由前壁。
- 检查出血后,取出布,敷了一些小肠周围的肾保持恰当的位置。用两个单丝缝合1关闭腹壁。用3-0丝线或不可吸收的单丝缝合线封闭皮肤。
- 通过仔细滴定去甲肾上腺素注射到猪已经被放置到俯卧位继续保持高于100毫米汞柱的收缩压。
- 术后程序
- 上述关腹后,保持温暖猪用加热垫和热循环毯。取出动脉线,用6-0普理灵施蒂希关闭穿刺孔在动脉并关闭切口部位。
- 打开猪到俯卧位,停止滴注去甲肾上腺素和断奶从呼吸机的猪。阿尔低小猪在其住房面积恢复并密切监测,以确保其从过程顺利恢复。采取的血液气体样品通过植入的颈静脉导管每个小时。提供乳酸林格氏液来代替体积和管理镇痛0.3毫克丁丙诺啡。
- 继拔管,密切监测猪直到它能够自发地喝。无人看管,直到它已经恢复了足够的意识,以保持胸骨斜卧不要让动物。不要返回已动过手术,以公司的其他动物,直到完全康复的动物。
4.手术后跟进
- 如果需要施用0.3毫克丁丙诺啡四每8小时为至少2天手术后或更长。在手术过程中经常给予抗生素单预防剂量。在感染迹象的情况下,管理头孢唑啉1克四每天两次和甲硝唑四每天一次,直到出现临床症状明显改善。辖乳酸林格氏液,直到猪喝足够的水。 1000 IU的肝素可用于锁定导管,以防止凝固。
- 经颈静脉导管和尿样品采集静脉血样来评估猪的临床症状,肾功能。
- 对于安乐死,诱导异丙酚IV(5-10毫升)猪的麻醉和异氟醚5%的维护。如上所述插管猪。 relaparatomy和肾组织样本收集后,由40 MVAL氯化钾静脉注射引起心脏骤停。
结果
在下文中,自体肾移植实验的结果(n = 4时)的证明。在移植肾检索后,猪收回他们的住房面积。同时,肾移植物在冰上储存为7小时35分钟(±18分钟)的平均时间。麻醉和重复剖腹手术的再诱导后,对侧肾脏切除和上述冷保存移植异位移植。从呼吸机断奶后,猪从手术恢复,并随访10天( 参见图1)。每日(1-4术后天;荚果),或每隔一天(6-10荚)血样收集进行血气分析;评估肾功能,血肌酐和尿素氮(BUN)值估计。为了对比,人们allotransplanted肾移植物的结果。对于免疫抑制,这只猪接受环孢素100毫克PO和合作rtisone 250毫克ivbid使用的外科技术是一样的,在自体移植的协议;无热缺血时间的应用。
所有的猪在随访期间均处于良好的临床状况。血清肌酐和尿素氮值显示为一天手术后的最高涨幅(克雷亚2.8±0.7毫克/分升,BUN 25.3±7毫克/分升),并下降至箱体10(克雷亚1.7±0.4毫克/分升,BUN 10.7±4毫克/分升)接近初始基准值。该allotransplanted移植肾表现出较高的肌酐和尿素氮值之后,良好的初始移植物功能,相比自体移植,很可能是由于拒绝时(图2 和图3)。酸碱止血( 图4)和电解质水平(图5)是稳定的,没有干预。组织学检查显示,自体移植的肾间质保存(FIGURË6),以及弥漫性间质炎症,小管炎和肾小球肾炎的肾脏allotransplanted(图7)。
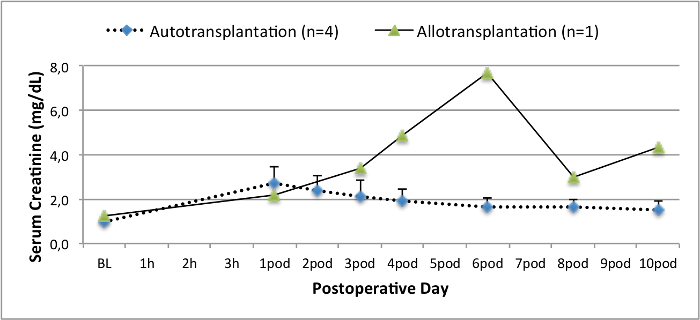
基线图2.血清肌酐值,血清肌酐值(均值和标准差)和10个手术。 请点击此处查看该图的放大版本。
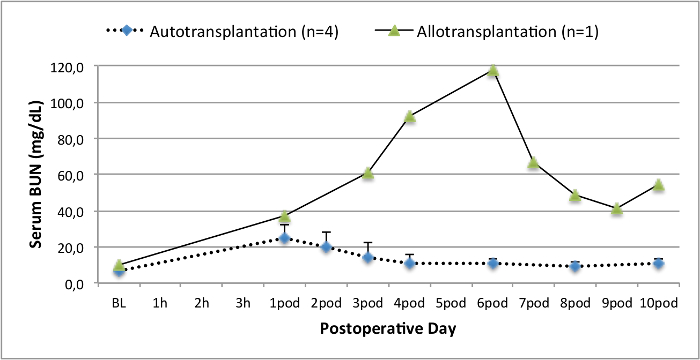
图3.血清尿素氮值,血清尿素氮值(均值和标准差)为基准10天手术后。 请点击此处查看该图的放大版本。
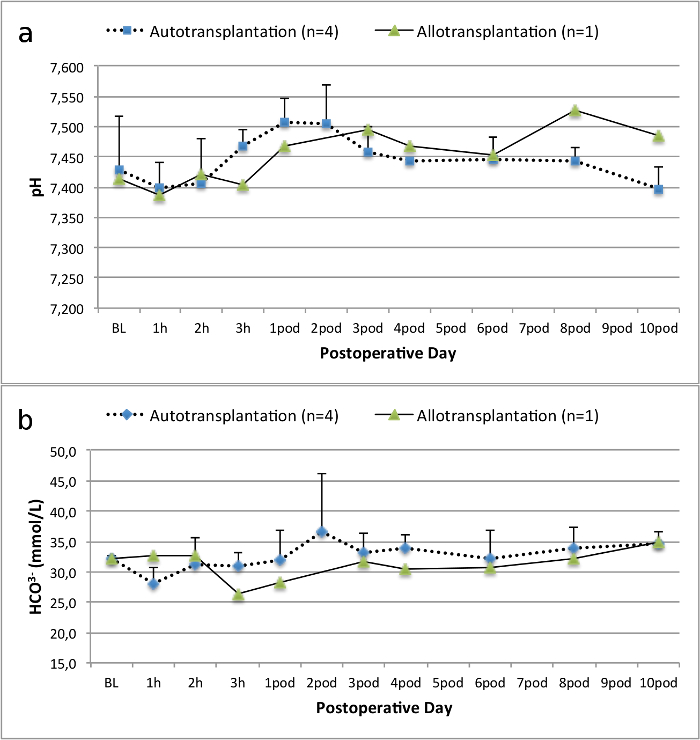
手术后图4.酸碱止血。酸碱止血(均值和标准差)为基线和10天。 请点击此处查看该图的放大版本。
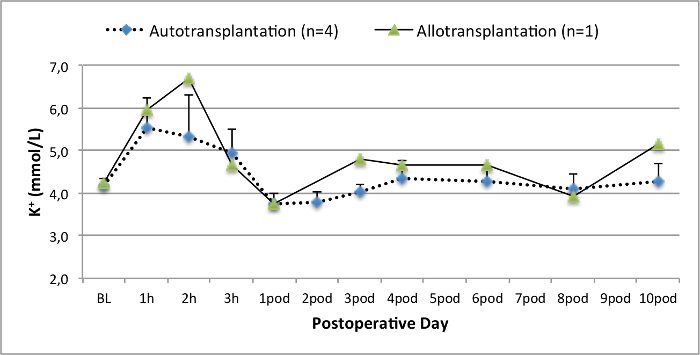
图5.电解质水平。电解质水平(均值和标准差)为基线和手术后10天。 请点击此处查看该图的放大版本。
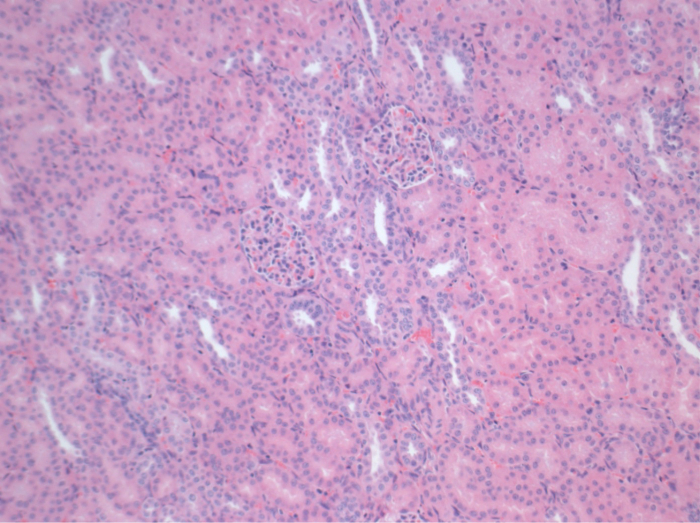
图6.组织学(H& E),放大100倍的自体移植肾间质正常手术后10天,请点击此处查看该图的放大版本。
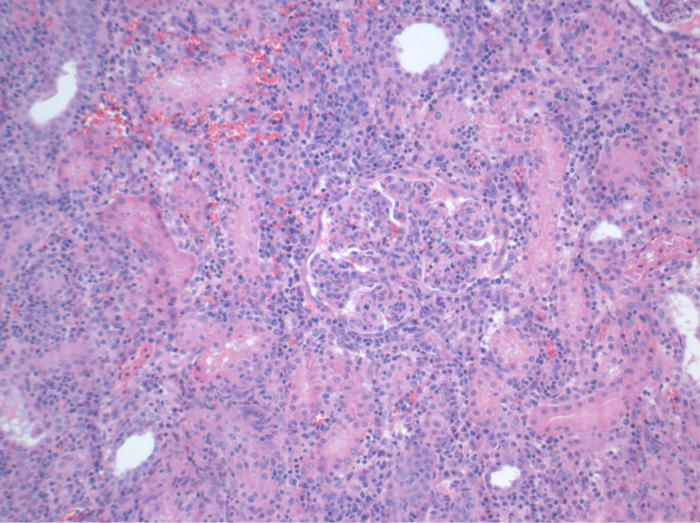
图7.组织学(H&E),放大100倍,广泛的间质炎症,小管炎和肾小球肾炎,具有抑制一致,在手术后10天allotransplanted肾。 请点击此处查看该图的放大版本。
讨论
猪肾移植模型提供了进一步的人类移植领域的一个独特的机会,由于在手术方面,生理学,生物化学和免疫学14相似之处。
根据实验研究的目的,自体肾移植的模型相比,同种移植模型的几个优点。虽然异体移植后28几组报告好移植肾功能,免疫猪是具有挑战性的,尤其是在肾移植。术前血液样本进行分析,以确保猪白细胞抗原(SLA)的兼容性是可行的,但价格昂贵,不切实际14。术后,提出免疫抑制剂如他克莫司和环孢霉素(钙调磷酸酶抑制剂,CNI)口服给药或静脉28。口服给药是不切实际的,因为猪通常拒绝吞咽口服药物学化。此外,肠梗阻可能避免免疫抑制药物和治疗药物水平维持足够的吸收。 CNI的IV在活跃动物连续输注技术要求高。静脉推注施用导致高的峰值,导致毒性。因此,对于新的保存技术的调查,自体肾移植的模型有几个优点。在上述演示的allotransplantated肾移植,肌酸酐和BUN的延迟和增加的峰值的代表性结果表明抑制,其通过组织学评估证实。
自体移植的猪模型先前已用于研究新的保鲜技术14,18,29。然而,在一个心脏跳动情形自体移植的猪的报道术后血清肌酸酐和BUN值的变化很大,取决于实验系统22,30上。我们在座的心脏跳动的捐赠协议,导致2.8毫克/分升(±0.7)和25.3毫克/分升BUN峰(±7.4)低术后血清肌酐高峰。这些结果与由ハント和同事28和 Snoeijs和同事31呈现的低峰值相媲美。
为了确保在猪自体移植模型肾移植后成功的结果,我们已经确定了几个关键的技术因素,最大限度地减少某些并发症的发生率。相比威斯康星州(UW)解决方案时,大学使用的组氨酸,色氨酸酮戊二酸溶液(HTK)减少血管痉挛的风险,由于钾的含量较低。进一步降低在再灌注点血管痉挛的危险,维拉帕米可注入肾动脉,和罂粟碱可局部检索期间和再灌注后给药。另外,去甲肾上腺素的连续滴注滴定以保持收缩期血压高于100毫米汞柱,确保均匀的灌注。它是保持至少直到猪被定位容易发生这种血压有用。此外,移植移植物的定位是很重要的,以防止新吻合血管的扭结。因此,它是有帮助的对侧左肾切除缝合移植物的吻合,以避免大量的机械操作之前。整理输尿管吻合术,包小肠周围的移植移植术后固定腹壁关闭后其位置。并发症,如因肠扭结肠梗阻很少观察到,但可能会导致严重的并发症,包括肠梗阻,肠穿孔和死亡。总体而言,精确的手术技术,麻醉周到,密切监测随访期间确保良好的临床疗效和移植物功能。
动脉和静脉吻合,可以PERFO使用不同的技术Rmed指。移植物的原位放置允许肾动脉和静脉端 - 端吻合。在异位移植的情况下,该接枝可定位在对侧肾窝为端 - 端吻合术,到髂血管,或直接在远端主动脉。与吻合异位移植到主动脉和静脉直接在端-端技术是优选的在此模型中,因为它可以降低血栓形成和血管痉挛32的风险。具有非常早静脉分叉解剖变异可能导致需要缝制两个单独静脉吻合。如果动脉或静脉相对较短,接枝可旋转180°以获得所述容器的长度。输尿管一侧到侧吻合可以取得良好的实验结果没有复杂狭窄或泌尿泄漏。
一般情况下,相对于其他动物模型肾移植的猪模型提供了优势。为D上述旁切,猪和人的设置,这使得新的技术相对快速翻译到临床实践之间存在着一定的相似性。相比啮齿动物模型中移植的技术在技术上是容易。此外,通过静脉导管的安置,外周血样品可以容易地收集并进行进一步的调查处理。尿液收集允许肾损伤和功能的进一步评估。收集尿液样品,经皮导管可以插入膀胱。为了避免操作由猪,远端应皮下隧道传送到动物的背部。用于收集尿液的另一个选择是利用代谢笼,这允许延长收集期间来估计在尿中的肌酸酐清除率和另外的生物标记的浓度。超声,CT扫描和MRI图像是可能的。循环后死亡捐赠协议可以通过应用来温暖模仿缺血以取回之前。此外,猪都比较容易处理,如果被阉割,以限制他们的攻击行为。
缺点包括动物采购,住房,手术等医疗设备和人力的高成本。这些因素意味着它是不可行的包括各研究组在大量的动物。此外,相比于啮齿动物模型,引用有限数量的在文献中为猪规范生物数据可用。作为新开发的技术,如新颖的保存方法的评估的一个替代方案中,其它基团所描述的常温离体灌注作为替代肾移植33,34。这种技术是更容易执行,更便宜。然而,标准化的肾移植体的移植提供了更多的类似于临床实践的模式,并允许较长的后续周期。因此,服务于一个更现实的评估移植换货。
总之,异位自体肾移植的猪模型提供了探讨移植肾成果的改进创新新方法临床重要的场景。特别是,该协议提供重要的技术细节,这将有利于成功建立自体肾移植模型,并允许新的调查结果的临床试验的快速翻译。
披露声明
The authors have nothing to disclose.
致谢
We thank the Sorin Group (Milano, Italy), XVIVO Perfusion Inc. (Goteborg, Sweden), and Braun AG (Melsungen, Germany) for their support. We highly appreciate the support of the John David and Signy Eaton Foundation.
材料
| Name | Company | Catalog Number | Comments |
| Anesthesia Equipment | |||
| Anesthesia Machine, Optimax | Moduflex Anesthesia Equipment | SN5180 | |
| Infusion Pump 3,000 | SIMS Graseby LTD. | SN300050447 | |
| Infusion Pump Line | Smith Medical ASD Inc. | 21-0442-25 | |
| Intravenous permanent catheter (9.5 Fr) | Cook Medical Company | G01865 | |
| Isoflurane Vapor 19.1 | Draeger Medical Canada Inc. | N/A | |
| Mallinckrodt, Tracheal Tube, 6.5 mm | Covidien Canada | 86449 | |
| Temperature Therapy Pad | Gaymar Industries Inc | TP26E | |
| Ventilator, AV 800 | DRE Medical Equipment | 40800AVV | |
| Warm Touch, Patient Warming System | Nellcor/ Covidien Canada | 5015300A | |
| Name | Company | Catalog Number | Comments |
| Surgical Equipment | |||
| Abdominal Retractor | Medite GmbH | 07-0001-00 | |
| Aorta/vein punch 4.0 mm, round | Scanlan International Inc. | 1001-602 | |
| De Bakey, Atraumatic Peripheral, Clamp | Aesculap Inc. | FB463R | |
| De Bakey-Beck, Atraumatic Vena Cava, Clamp | Aesculap Inc. | FB519R | |
| De Bakey, Atraumatic Mini-Bulldog, Straight | Aesculap Inc. | FB422R | |
| De Bakey, Atraumatic Mini-Bulldog, Curved | Aesculap Inc. | FB423R | |
| De Bakey, Atraumatic Coarctation Clamp, Angled | Aesculap Inc. | FB453R | |
| Dissection Blade #11 | Feather Safety Razor Co. | 089165B | |
| Connector (1/4") with male luer lock | Sorin Group Inc. | AB1452 | |
| Liver Admin Set (flush line) | CardioMed Supplies Inc | 17175 | |
| Maxon, 1 | Covidien Canada | 606173 | |
| Med-Rx Suction Connecting Tube | Benlan Inc. | 70-8120 | |
| Organ Bag | CardioMed Supplies Inc | 2990 | |
| Potts – De Martel, Scissors | Aesculap Inc. | BC648R | |
| Renal artery cannula, 1.6" | Sorin Group Inc. | VC-11000 | |
| Sofsilk, 2-0 | Covidien Canada | S405 | |
| Sofsilk, 3-0 | Covidien Canada | S404 | |
| Satinsky, Suprahepatic Cava Clamp | Aesculap Inc. | FB605R | |
| Suction Tip | Tyco Healthcare Group LP | 8888501023 | |
| Surgipro II, 6-0 | Covidien Canada | VP733X | |
| Valleylab, Cautery Pencil | Covidien Canada | E2515H | |
| Valleylab, Force Tx | Valleylab Inc. | 216151480 | |
| Valleylab, Patient Return Electrode | Covidien Canada | E7507 | |
| Name | Company | Catalog Number | Comments |
| Medication | |||
| Atropine Sulfate 15 mg/30 ml | Rafter 8 Products | 238481 | |
| Buprenorphine 0.3 mg/ml | RB Pharmaceuticals LDT | N/A | |
| Ceftiofur 3 mg/ml | Pfizer Canada Inc. | 11103 | |
| Cefazolin 1 g | Pharmaceutical Partners of Canada Inc. | 2237138 | |
| Fentanyl Citrate 0.25 mg/5 ml | Sandoz Canada Inc. | 2240434 | |
| Heparin 10,000 iU/10 ml | Sandoz Canada Inc. | 10750 | |
| Histidine-tryptophan-ketoglutarate (HTK) solution | Methapharm | CU001LBG | |
| Isoflurane 99.9%, 250 ml | Pharmaceutical Partners of Canada Inc. | 2231929 | |
| Ketamine Hydrochloride 5,000 mg/50 ml | Bimeda-MTC Animal Health Inc. | 612316 | |
| Lactated Ringer’s + 5% Dextrose 1 L | Baxter Corporation | JB1064 | |
| Lactated Ringer’s 1 L | Baxter Corporation | JB2324 | |
| Metronidazole 500 mg/100 ml | Baxter Corporation | 870420 | |
| Midazolam 50 mg/10 ml | Pharmaceutical Partners of Canada Inc. | 2242905 | |
| Norepinephrine 16 mg/250 ml Dextrose 5% | Baxter Corporation | N/A | |
| Pantoprazole 40 mg | Sandoz Canada Inc. | 2306727 | |
| Papaverine 65 mg/2 ml | Sandoz Canada Inc. | 9881 | |
| Propofol 1,000 mg/100 ml | Pharmascience Inc. | 2244379 | |
| Saline 0.9%, 1 L | Baxter Corporation | 60208 | |
| Solu-Medrol 500 mg | Pfizer Canada Inc. | 2367963 | |
| Verapamil | Sandoz Canada Inc. | 2166739 | |
| Xylocaine Endotracheal 10 mg/50 ml | AstraZeneca | 2003767 |
参考文献
- Wolfe, R. A., Ashby, V. B., et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 341 (23), 1725-1730 (1999).
- Ingsathit, A., Kamanamool, N., Thakkinstian, A., Sumethkul, V. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta-analysis. Transplantation. 95 (7), 943-948 (2013).
- Tonelli, M., Wiebe, N., et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 11 (10), 2093-2109 (2011).
- Matas, A. J., et al. OPTN/SRTR Annual Data Report 2012: Kidney. Am J Transplant. 14, (2014).
- . Annual Report 2013 - Eurotransplant International Foundation. Available from: https://www.eurotransplant.org/cms/mediaobject.php?file=AR20135.pdf (2013)
- Morrissey, P. E., Monaco, A. P. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 97 (3), 258-264 (2014).
- Maggiore, U., Oberbauer, R., et al. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant. 30 (2), 217-222 (2014).
- Summers, D. M., Johnson, R. J., Hudson, A., Collett, D., Watson, C. J., Bradley, J. A. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. 381 (9868), 727-734 (2013).
- Wadei, H. M., Heckman, M. G., et al. Comparison of kidney function between donation after cardiac death and donation after brain death kidney transplantation. Transplantation. 96 (3), 274-281 (2013).
- Moers, C., Smits, J. M., et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 360 (1), 7-19 (2009).
- Pereira-Sampaio, M. A., Favorito, L. A., Sampaio, F. J. B. Pig kidney: anatomical relationships between the intrarenal arteries and the kidney collecting system. Applied study for urological research and surgical training. J Urol. 172 (5 Pt 1), 2077-2081 (2004).
- Bagetti Filho, H. J. S., Pereira-Sampaio, M. A., Favorito, L. A., Sampaio, F. J. B. Pig kidney: anatomical relationships between the renal venous arrangement and the kidney collecting system. J Urol. 179 (4), 1627-1630 (2008).
- Giraud, S., Favreau, F., Chatauret, N., Thuillier, R., Maiga, S., Hauet, T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol. 2011 (21), 532127 (2011).
- Gallinat, A., Paul, A., et al. Role of oxygenation in hypothermic machine perfusion of kidneys from heart beating donors. Transplantation. 94 (8), 809-813 (2012).
- Thuillier, R., Allain, G., et al. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J Surg Res. 184 (2), 1174-1181 (2013).
- Hosgood, S. A., Barlow, A. D., Yates, P. J., Snoeijs, M. G. J., van Heurn, E. L. W., Nicholson, M. L. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J Surg Res. 171 (1), 283-290 (2011).
- Delpech, P. O., Thuillier, R., et al. Effects of warm ischaemia combined with cold preservation on the hypoxia-inducible factor 1α pathway in an experimental renal autotransplantation model. Br J Surg. 101 (13), 1739-1750 (2014).
- Kirk, A. D. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev. 196, 176-196 (2003).
- Golriz, M., Hafezi, M., et al. Do we need animal hands-on courses for transplantation surgery. Clin Transplant. 27, 6-15 (2013).
- He, B., Musk, G. C., Mou, L., Waneck, G. L., Delriviere, L. Laparoscopic surgery for kidney orthotopic transplant in the pig model. JSLS. 17 (1), 126-131 (2013).
- Faure, A., Maurin, C., et al. An experimental porcine model of heterotopic renal autotransplantation. Transplant Proc. 45 (2), 672-676 (2013).
- Hosgood, S. A., Yates, P. J., Nicholson, M. L. 1400W reduces ischemia reperfusion injury in an ex-vivo porcine model of the donation after circulatory death kidney donor. World J Transplant. 4 (4), 299-305 (2014).
- Ghanekar, A., Mendicino, M., et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 172 (9), 5693-5701 (2004).
- Ghanekar, A., Lajoie, G., et al. Improvement in rejection of human decay accelerating factor transgenic pig-to-primate renal xenografts with administration of rabbit antithymocyte serum. Transplantation. 74 (1), 28-35 (2002).
- Cowan, P. J., Cooper, D. K. C., d'Apice, A. J. F. Kidney xenotransplantation. Kidney Int. 85 (2), 265-275 (2014).
- Seldinger, S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 39 (5), 368-376 (1953).
- Hanto, D. W., Maki, T., et al. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 10 (11), 2421-2430 (2010).
- Maathuis, M. -. H. J., Manekeller, S., et al. Improved kidney graft function after preservation using a novel hypothermic machine perfusion device. Ann Surg. 246 (6), 982-991 (2007).
- Gallinat, A., Paul, A., et al. Hypothermic reconditioning of porcine kidney grafts by short-term preimplantation machine perfusion. Transplantation. 93 (8), 787-793 (2012).
- Snoeijs, M. G., Matthijsen, R. A., et al. Autologous transplantation of ischemically injured kidneys in pigs. J Surg Res. 171 (2), 844-850 (2011).
- Golriz, M., Fonouni, H., Nickkholgh, A., Hafezi, M., Garoussi, C., Mehrabi, A. Pig kidney transplantation: an up-to-date guideline. Eur Surg Res. 49 (3-4), 121-129 (2012).
- Hosgood, S. A., Bagul, A., Yang, B., Nicholson, M. L. The relative effects of warm and cold ischemic injury in an experimental model of nonheartbeating donor kidneys. Transplantation. 85 (1), 88-92 (2008).
- Hoyer, D. P., Gallinat, A., et al. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation. 98 (9), 944-950 (2014).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。