Citometría de flujo y clasificación de células activadas por fluorescencia (FACS): Aislamiento de linfocitos B esplénicos
Fuente: Perchet Thibaut1,2,3, Meunier Sylvain1,2,3, Sophie Novault4, Rachel Golub1,2,3
1 Unidad de Linfopoyesis, Departamento de Inmunología, Instituto Pasteur, París, Francia
2 INSERM U1223, París, Francia
3 Université Paris Diderot, Sorbonne Paris Cité, Cellule Pasteur, París, Francia
4 Flow Cytometry Platfrom, Citometría y Biomarcadores UtechS, Centro de Ciencias Traslacionales, Instituto Pasteur, París, Francia
La función general del sistema inmunológico es defender el cuerpo contra organismos infecciosos y otros invasores. Los glóbulos blancos, o leucocitos, son los actores clave del sistema inmunitario. Tras la infección, se activan e inician una respuesta inmunitaria. Los leucocitos se pueden dividir en varias subpoblaciones (por ejemplo, células mieloides, linfocitos, células dendríticas) basándose en diferentes parámetros que pueden ser biológicos, físicos y/o funcionales (por ejemplo, tamaño, granularidad y secreción). Una forma de caracterizar a los leucocitos es a través de sus proteínas superficiales, que son principalmente receptores. Cada población de leucocitos expresa una combinación específica de receptores (por ejemplo, citotóxicos, activadores, receptores de migración) que pueden definir subconjuntos entre las poblaciones. Como el sistema inmunitario abarca una amplia gama de poblaciones celulares, es esencial caracterizarlas para descifrar su participación en la respuesta inmunitaria.
La citometría de flujo (FC o FCM) es un método ampliamente utilizado para analizar la expresión de la superficie celular y las moléculas intracelulares, caracterizando y definiendo diferentes tipos de células en una mezcla de células heterogéneas. Los citometros de flujo se componen de tres subsistemas principales: fluidos, ópticos y electrónicos. El sistema de fluidos transporta las células en una corriente de tal manera que pasan delante de un láser uno por uno. El sistema óptico consta de fuentes de luz (láser) para iluminar las partículas, filtros ópticos para dirigir la luz resultante y señales fluorescentes a los detectores adecuados. Por último, el sistema electrónico convierte las señales de luz detectadas en señales electrónicas que pueden ser procesadas por el ordenador. A medida que una célula individual pasa delante del rayo láser, dispersa la luz. Un detector delante del haz mide la dispersión hacia adelante (FS) y varios detectores hacia el lado de la dispersión lateral (SC). El FS se correlaciona con el tamaño de celda y SC es proporcional a la granularidad de las células. De esta manera, las poblaciones celulares a menudo se pueden distinguir en función de las diferencias en su tamaño y granularidad solamente.
Además de analizar el tamaño, la forma y la complejidad de una célula, la citometría de flujo se utiliza ampliamente para detectar la expresión de los receptores de superficie celular (1). Esto se logra mediante el uso de anticuerpos monoclonales etiquetados con fluorocromo que se unen a receptores específicos de células conocidos. Tras la excitación, estos fluorocromos enlazados emiten una luz de longitud de onda específica, llamada longitud de onda de emisión, que puede ser detectada y puntuada. Las mediciones de fluorescencia proporcionan datos cuantitativos y cualitativos sobre los receptores de superficie celular etiquetados con fluorocromo. Los hematólogos utilizaron por primera vez la FC para el seguimiento terapéutico de las poblaciones de células inmunitarias (2). Ahora, se utiliza para una amplia gama de aplicaciones tales como inmunofenotipado, viabilidad celular, expresión génica, recuento celular y análisis de GFP.
FACS (Fluorescent Activated Cell Sorter) es un tipo especializado de citometría de flujo, que ordena una población de células en subpoblación utilizando etiquetado fluorescente. Al igual que la citometría de flujo convencional, se recopilan los primeros datos FS, SC y fluorescentes. A continuación, la máquina aplica una carga (negativa o positiva) y un sistema de desviación electrostática (electroimanes) facilita la recolección de gotas cargadas que contienen células en tubos apropiados.
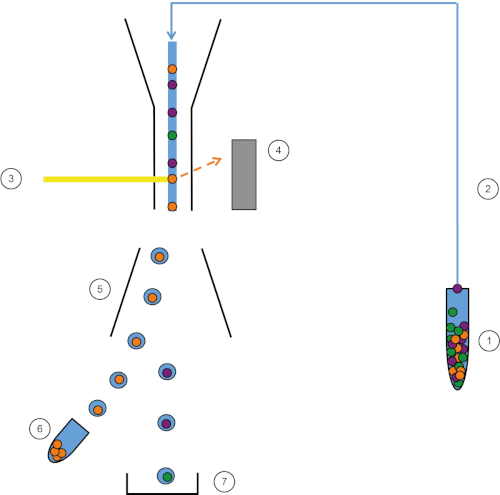
Figura 1: Representación esquemática de FACS. La muestra (1) se aspira en el FACS (2) y se pasa delante del láser (3). La fluorescencia celular es sintensada por detectores de fluorescencia (4). Finalmente, las células se incorporan en gotas y las células de interés son desviadas por placas de desviación (5) y recogidas en un tubo de recolección (6). Las celdas restantes entran en la basura (7). Haga clic aquí para ver una versión más grande de esta figura.
El aspecto de clasificación del FACS presenta muchas ventajas. Muchas pruebas pueden ayudar a entender el papel de las células específicas en el sistema inmunitario, como los análisis de la expresión génica como RT-qPCR, ciclo celular o secreción de citoquinas. Sin embargo, las células deben purificarse aguas arriba para obtener resultados claros y específicos. Aquí, FACS viene en útil y las células deseadas se pueden clasificar con gran pureza, produciendo resultados altamente confiables y reproducibles. FacS también se puede utilizar para clasificar las células en función de la tinción nuclear u otra intracelular y de acuerdo con la presencia, ausencia y densidad de los receptores de superficie. FACS es ahora una técnica estándar para la purificación de subpoblaciones de células y tiene la capacidad de ordenar hasta cuatro poblaciones simultáneamente.
Este ejercicio de laboratorio demuestra cómo aislar leucocitos esplénicos y luego cómo clasificar específicamente las células linfoides B de la mezcla de células de leucocitos esplénicos utilizando FACS.
1. Preparación
- Antes de comenzar, ponte guantes de laboratorio y la ropa protectora adecuada.
- Esterilice todas las herramientas de disección, primero con un detergente y luego con 70% de etanol y luego seque bien.
- Preparar 50 ml de la solución salina equilibrada de Hank (HBSS) que contiene un 2% de suero de becerro fetal (FCS).
2. Disección
- Usando un sistema de administración de dióxido de carbono, eutanasia el rat
En este protocolo, purificamos linfocitos B esplénicos utilizando la tecnología FACS. Primero aislamos leucocitos del bazo y los manchamos. Usando una combinación de marcadores de superficie de celda B, creamos una estrategia de gating para ordenarlos (Figura 2, panel superior). Al final del experimento verificamos si las células en el tubo de recolección eran células B a través de una "prueba de pureza". Mantuvimos la misma estrategia de gating y observamos que más del 98% de las...
La citometría de flujo es una técnica de primera mano para caracterizar y clasificar las poblaciones de células inmunitarias con un alto grado de pureza. Es una herramienta primordial en el campo de la investigación, ya que permite el enriquecimiento de poblaciones celulares específicas y para descifrar la respuesta inmune a los patógenos. Con el aumento en el número de fluorocromos y citómetros disponibles, el número de parámetros detectables se incrementa considerablemente. Como resultado, el análisis bioinf...
- Lanier, L. L. Just the FACS. The Journal of Immunology, 193 (5), 2043-2044 (2014).
- Walker, J. M. Epiblast Stem Cells IN Series Editor.
- Tung, J. W., Heydari, K., Tirouvanziam, R., Sahaf, B., Parks. D. R., Herzenberg, L. A., and Herzenberg. L. A. Modern Flow Cytometry: A Practical Approach. Clinics in Laboratory Medicine. 27 (3), 453-468 (2007).
- Walker, J. M. Tumor Angiogenesis Assays IN Series Editor.
Saltar a...
Vídeos de esta colección:

Now Playing
Citometría de flujo y clasificación de células activadas por fluorescencia (FACS): Aislamiento de linfocitos B esplénicos
Immunology
91.4K Vistas

Clasificación celular activada magnéticamente (MACS): Aislamiento de linfocitos T del timo
Immunology
22.2K Vistas

Ensayos ELISA: Indirecto, Sándwich y Competitivo
Immunology
233.0K Vistas

Ensayo ELISPOT: Detección de esplenocitos secretores de IFN-γ
Immunology
27.8K Vistas

Inmunohistoquímica e Inmunocitoquímica: Imágenes de tejidos a través de microscopía óptica
Immunology
77.3K Vistas

Generación de anticuerpos: Producción de anticuerpos monoclonales mediante hibridomas
Immunology
42.6K Vistas

Microscopía de Inmunofluorescencia: Tinción de inmunofluorescencia de secciones de tejido incrustado en parafina
Immunology
52.7K Vistas

Microscopía de Fluorescencia Confocal: Una Técnica para Determinar la Localización de Proteínas en Fibroblastos de Ratón
Immunology
42.4K Vistas

Técnicas basadas en inmunoprecipitación: purificación de proteínas endógenas mediante perlas de agarosa
Immunology
86.7K Vistas

Análisis del ciclo celular: Evaluación de la proliferación de células T CD4 y CD8 después de su estimulación mediante tinción CFSE y citometría de flujo
Immunology
23.7K Vistas

Transferencia celular adoptiva: Introducción de cenocitos de un ratón donante a un ratón huésped y evaluación del éxito a través de FACS
Immunology
21.6K Vistas

Ensayo sobre la muerte celular: Ensayo de liberación de cromo de la capacidad citotóxica
Immunology
151.1K Vistas
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados