Un abonnement à JoVE est nécessaire pour voir ce contenu. Connectez-vous ou commencez votre essai gratuit.
Method Article
Ablation de la tige oculaire pour augmenter la maturation ovarienne chez les crabes de vase
Dans cet article
Erratum Notice
Résumé
Deux protocoles d’ablation du pédoncule oculaire (c.-à-d. des approches de cautérisation et de chirurgie) ont été effectués sur des crabes femelles anesthésiés. L’ablation oculaire des crabes de vase a accéléré la maturation des ovaires sans diminuer le taux de survie.
Résumé
Les crabes de vase (Scylla spp.) sont des espèces de crustacés commercialement importantes que l’on trouve dans toute la région indo-pacifique occidentale. Pendant la culture, l’induction de la maturation ovarienne est importante pour répondre à la demande des consommateurs de crabes de vase matures et accélérer la production de graines. L’ablation de la tige oculaire est un outil efficace pour améliorer la maturation ovarienne chez les crabes de boue. Cependant, il n’existe pas de protocole standard pour l’ablation du pédoncule oculaire des crabes de vase. Dans cette étude, deux techniques d’ablation du pédoncule oculaire sont décrites : la cautérisation (l’utilisation de métal chaud pour ablater le pédoncule oculaire d’un crabe anesthésié) et la chirurgie (l’ablation du pédoncule oculaire à l’aide de ciseaux chirurgicaux). Avant l’ablation du pédoncule oculaire, les femelles sexuellement matures (CW > 86 mm) ont été anesthésiées à l’aide d’un sac de glace (−20 °C) avec de l’eau de mer. Lorsque la température de l’eau a atteint 4 °C, le sac de glace a été retiré de l’eau. L’eau de mer qui coule (température ambiante : 28 °C) a été utilisée pour récupérer de l’anesthésie immédiatement après l’ablation du pédon. La mortalité ne s’est pas produite pendant ou après le processus d’ablation du pédoncule oculaire. Le protocole d’ablation du pédoncule oculaire présenté ici a accéléré la maturation ovarienne des crabes de boue.
Introduction
Les quatre espèces de crabes de vase appartenant au genre Scylla sont des espèces de crustacés commercialement importantes en aquaculture 1,2. La croissance des crustacés, y compris les crabes de vase, et leur transformation de la phase prématurée (subadulte ou pubertaire) à la phase sexuellement mature (adulte) se produisent par un processus de mue qui implique l’excrétion périodique d’exosquelettes plus anciens et plus petits. La largeur de la carapace (CW), les chélipèdes et les morphologies des lambeaux abdominaux sont largement utilisés pour déterminer la maturité sexuelle de Scylla spp. 3,4,5. Le processus de mue est régulé par l’action de diverses hormones et nécessite une énorme quantité d’énergie6. En plus du processus normal de mue, la perte de membres, volontaire ou induite par des facteurs externes, accélère la mue des crabes sans affecter leur taux de survie 7,8,9. Par conséquent, l’autotomie des membres est couramment utilisée pour l’induction de la mue dans l’industrie de l’élevage du crabe de vaseà carapace molle 7,9.
L’ablation unilatérale ou bilatérale de la tige oculaire est surtout populaire chez les crevettes d’eau douce et les crevettes marines pour la maturation des gonades et la production de graines10,11,12,13. Les techniques courantes d’ablation du pédoncule oculaire chez les crustacés sont les suivantes: (i) ligature à la base du pédoncule oculaire à l’aide d’une ficelle14,15; ii) cautérisation du pédoncule oculaire à l’aide de pinces chaudes ou d’appareils d’électrocautérisation16; iii) l’ablation ou le pincement direct du pédoncule oculaire pour laisser une plaie ouverte12; et (iv) l’ablation du contenu de la tige oculaire par incision après avoir tranché la partie distale de l’œil avec un rasoir17. Les organes X du pédoncule oculaire sont des organes endocriniens importants chez les crustacés car ils régulent les hormones hyperglycémiques des crustacés (CHH), les hormones inhibitrices de la mue (MIH) et les hormones inhibitrices de la vitellogenèse (VIH)6,18,19,20,21,22. Les organes X de la tige oculaire (ou le complexe des glandes sinusales) synthétisent et libèrent des hormones inhibitrices des gonades (GIH), également connues sous le nom d’hormones inhibant la vitellogenèse (VIH), appartenant à la famille des hormones neuropeptidiques6. L’ablation unilatérale ou bilatérale du pédoncule oculaire réduit la synthèse de la GIH, ce qui entraîne la dominance des hormones stimulantes (c.-à-d. les hormones stimulant les gonades, GSH) et l’accélération du processus de maturation ovarienne chez les crustacés23,24,25,26. Sans l’influence de la GIH après l’ablation de la tige oculaire, les crustacés femelles consacrent leur énergie au développement des ovaires27. Il a été constaté que l’ablation unilatérale du pédoncule oculaire est suffisante pour l’induction de la maturation ovarienne chez les crustacés11 et que le pédoncule oculaire ablé des crevettes et des crabes peut se régénérer après plusieurs mues28. Quatre stades de développement ovarien ont été enregistrés chez Scylla spp. : i) immature (stade 1), ii) maturation précoce (stade 2), iii) prématuration (stade 3) et iv) pleine maturité (stade 4)29,30. Le stade ovarien immature se trouve chez les femelles immatures. Après la mue pubertaire et l’accouplement, l’ovaire immature commence à se développer et finit par mûrir (stade 4) avant de frayer31.
Un protocole d’ablation du pédoncule oculaire est essentiel au développement du stock de géniteurs de crabe de vase et à la production de semences. Sur le marché alimentaire mondial, les crabes de vase matures avec des ovaires complètement matures (stade 4) plutôt que les crabes avec une teneur musculaire plus élevée sont préférés par les consommateurs et, par conséquent, ont une valeur commerciale plus élevée, même plus élevée que les gros mâles. Il n’existe pas de protocole complet pour l’ablation du pédoncule oculaire des crabes de boue. Le protocole d’ablation du pédoncule oculaire dans ce travail minimise le stress en utilisant des crabes entièrement anesthésiés et minimise les blessures physiques au personnel causées par les morsures de crabe. Ce protocole est simple et rentable. Ici, nous présentons un protocole pour l’ablation du pédoncule oculaire de Scylla spp. qui peut induire la maturation de la gonade. Deux techniques d’ablation de la tige oculaire (cautérisation et chirurgie) ont été testées et leur efficacité comparée en fonction du taux de développement gonadique des crabes de boue femelles.
Protocole
Ce protocole est conforme au Code de pratiques malaisien pour le soin et l’utilisation des animaux à des fins scientifiques décrit par l’Association malaisienne des sciences des animaux de laboratoire. Le sacrifice des échantillons expérimentaux a été effectué conformément au National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, révisé en 1978). Des crabes de vase femelles sexuellement prématurés (crabe de boue orange S. olivacea) ont été récoltés sur le marché local (5°66′62′′N, 102°72′33′′E) dans les zones humides de Setiu en Malaisie. L’espèce de crabe de vase a été identifiée en fonction des caractéristiques morphologiques1.
1. Prélèvement et désinfection des échantillons
- Recueillir des crabes de vase femelles en santé, actives et prématurées (figure 1).
REMARQUE : Les crabes femelles prématurés ont des lambeaux abdominaux triangulaires et de couleur claire ainsi qu’une plage CW de 80 à 85 mm. - Lavez les crabes avec de l’eau du robinet chlorée (eau douce) pour enlever les débris et les parasites osmophiles.
- Faire tremper les crabes dans du formaldéhyde de 150 ppm avec une salinité de 20 ppt pendant 30 min.
- Maintenir une aération continue et douce avec des pierres aériennes pendant le traitement au formaldéhyde. La source d’aération peut provenir d’une conduite d’aération centrale ou d’une pompe d’aérateur d’aquarium.
- Lavez les crabes avec de l’eau de mer qui coule pour éliminer tout formaldéhyde résiduel.
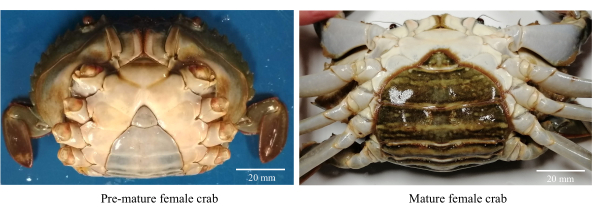
Figure 1 : Morphologie abdominale des crabes de vase femelles utilisés pour identifier les stades de maturation sexuelle. Veuillez cliquer ici pour voir une version agrandie de cette figure.
2. Acclimatation
- Transférer chaque femelle désinfectée dans un réservoir circulaire séparé de 32 L.
- Élever les femelles pendant 3 jours dans une salinité de 20 ppt et continuer à se nourrir deux fois par jour (matin 09h00 et soir 20h00) avec des poissons marins hachés à environ 4% à 5% du poids corporel du crabe.
- Enlevez les aliments excédentaires et non consommés en siphonnant avant l’alimentation du matin.
- Échangez 10% de l’eau de mer d’élevage du crabe (20 ppt) par jour.
3. Mue induite pour la maturité sexuelle
- Coupez toutes les jambes sauf les jambes de natation à l’aide de ciseaux stérilisés.
- Attrapez le crabe avec un filet à pelle et tenez-le soigneusement. Coupez d’abord les deux chélipèdes, puis les jambes de marche à la deuxième articulation à l’aide de ciseaux. Le crabe autotomisera automatiquement les appendices endommagés. L’anesthésie n’est pas nécessaire pour l’autotomie des membres.
- Laver le crabe dans de l’eau douce immédiatement après l’autotomie des membres.
- Transférer individuellement les crabes autotomisés dans des paniers en plastique perforés (28 cm L x 22 cm L x 7 cm H) et les placer dans un réservoir en fibre de verre (305 cm L x 120 cm L x 60 cm H).
NOTE: Deux paniers peuvent être attachés et clipsés ensemble. Le panier supérieur est utilisé comme couverture afin que le crabe ne puisse pas s’échapper du panier. - Utilisez un système d’aquaculture en recirculation (SAR) avec une salinité de 20 ppt et une profondeur d’eau d’au moins 10 cm pour vous assurer que tout le panier en plastique est submergé.
- Continuer à nourrir le crabe femelle autotomisé avec du poisson marin haché deux fois par jour à 5 % à 7 % du poids corporel du crabe.
- Élever les crabes jusqu’à maturité sexuelle par mue (35 jours).
REMARQUE : La mue induite peut être ignorée pour la maturation ovarienne commerciale et la production de graines avec des crabes de vase femelles matures sauvages. Les femelles matures récoltées dans la nature doivent être acclimatées et directement soumises à une anesthésie par choc dû au froid et à une ablation subséquente du pédoncule oculaire.
4. Anesthésie
- Sélectionnez les femelles sexuellement matures avec un lambeau abdominal ovale de couleur foncée avec un CW >86 mm (Figure 1).
- Attrapez les crabes avec un filet à pelle et gardez-les individuellement dans de petits aquariums pour l’anesthésie.
- Après 5 min de période d’acclimatation, ajouter du 2-phénoxyéthanol (2-PE) à 2 mL/L dans chaque aquarium et prévoir 15 min de traitement anesthésique.
- Assurez-vous que les crabes sont complètement anesthésiés par l’absence de mouvement spontané.
5. Ablation de la tige oculaire
- Technique de cautérisation
- Effectuez toutes les procédures sur une table et dans un espace ouvert.
- Prenez une tige métallique nickel-acier à tête plate (p. ex., un tournevis) avec un manche en bois ou en plastique et couvrez la poignée avec une serviette en coton humide.
- Stérilisez deux pinces chirurgicales inoxydables dans un autoclave.
- Préparez de l’éthanol à 70 % dans un flacon pulvérisateur et tenez-le à l’écart de toute source liée au feu, comme le chalumeau et le tournevis chauffé au rouge. Ayez du papier de soie prêt à l’emploi.
REMARQUE : L’éthanol est facilement inflammable. Maintenez une distance sécuritaire des sources d’incendie. - Connectez solidement un chalumeau à une bouteille de gaz (butane).
ATTENTION : Suivez les instructions sur le chalumeau et la bouteille de gaz. Assurez-vous que le chalumeau est éteint lors de la connexion avec la bouteille de gaz. Lisez et suivez toutes les précautions de sécurité incendie mentionnées sur la bouteille de gaz. - Portez des gants de coton épais pour éviter les blessures causées par des objets chauds.
- Soumettre l’extrémité de la tige métallique au feu du chalumeau jusqu’à ce que la tige métallique soit rouge vif.
- Couvrir le crabe anesthésié avec une serviette en coton humide.
REMARQUE : Couvrez les antennes du crabe pour éviter des dommages inutiles. - Tenez un œil du crabe avec des pinces stérilisées.
REMARQUE: Stériliser les pinces dans un autoclave pour la première utilisation et désinfecter avec de l’éthanol à 70% pour une utilisation ultérieure sur d’autres crabes. - Tenez la pointe plate en métal chauffé au rouge sur l’œil du crabe et appuyez légèrement pendant environ 10−15 s jusqu’à ce que le pédoncule de couleur orange ou orange rougeâtre. Soyez prudent lorsque vous effectuez cette étape pour éviter d’endommager les structures adjacentes.
REMARQUE: Deux personnes sont nécessaires pour effectuer l’ablation du pédoncule oculaire suivant la méthode de cautérisation: une pour tenir le crabe et une autre pour effectuer la procédure d’ablation. - Désinfectez les pinces avec un spray d’éthanol à 70% pour éviter toute contamination croisée entre les crabes.
REMARQUE: N’effectuez cette étape qu’au moins 5 minutes après la procédure d’ablation du pédoncule oculaire pour vous assurer que les pinces sont refroidies avant la désinfection à l’aide d’éthanol à 70% pour prévenir les risques d’incendie potentiels. - Après avoir effectué l’ablation du pédoncule oculaire sur tous les crabes, trempez la tige métallique en acier nickel chaud (tournevis) dans l’eau du robinet.
- Désinfectez la serviette avant de la réutiliser. Plusieurs serviettes peuvent être utilisées pour gagner du temps.
REMARQUE: Lavez la serviette avec de l’eau du robinet et trempez-la dans de l’eau chlorée de 30 ppm pendant 5 min. Ensuite, lavez à nouveau la serviette à l’eau du robinet et trempez-la dans une solution de thiosulfate de sodium à 1 g/L. - Gardez le chalumeau dans un endroit sûr après l’avoir éteint et attendez qu’il revienne à la température ambiante (environ 30 minutes) avant de le déconnecter.
- Technique chirurgicale
- Effectuez la procédure dans un endroit bien ventilé.
- Stériliser deux ciseaux chirurgicaux et des pinces dans un autoclave.
- Versez 50 mL d’éthanol à 70 % dans un bécher en verre de 100 mL.
- Portez des gants de coton épais.
- Tenez le crabe anesthésié et couvrez-le d’une serviette en coton humide.
- Tenez un œil du crabe avec des pinces stérilisées.
- Coupez rapidement la tige oculaire à l’aide de ciseaux chirurgicaux stérilisés.
NOTE: L’hémolymphe peut être perdue de la partie blessée du crabe. - Trempez les ciseaux et les pinces dans de l’éthanol à 70% après chaque utilisation et séchez-les avec du papier de soie avant de les réutiliser.
6. Soins post-anesthésiques
- Préparer de l’eau de mer filtrée à 20 ppt et conserver dans un réservoir supérieur avec une aération continue.
- Connectez un tuyau flexible avec le réservoir aérien pour l’écoulement gravitationnel de l’eau.
- Immédiatement après l’ablation du pédon, placez le crabe dans le panier et soumettez le crabe à l’eau de mer qui coule (température ambiante de l’eau : 28 °C) du réservoir supérieur.
- Maintenez l’eau de mer en écoulement et surveillez le crabe jusqu’à ce qu’il puisse se déplacer spontanément, ce qui indique une récupération de l’anesthésie.
REMARQUE: L’eau de mer peut être préparée dans un réservoir souterrain et une pompe à eau submersible peut être utilisée pour l’écoulement de l’eau. - Gardez les crabes individuellement dans de l’eau de mer de 20 ppt avec aération dans un aquarium pendant 30 minutes pour une observation plus approfondie.
REMARQUE : Les crabes récupérés seront élevés individuellement dans le cadre du processus subséquent d’élevage des géniteurs.
7. Observation de la maturation ovarienne
- Élevage de géniteurs
- Transférer les crabes matures dans des bassins circulaires individuels de 32 L.
- Continuez à nourrir avec des poissons marins hachés (congelés à −20 °C) deux fois par jour (matin 09h00 et soir 20h00), et retirez les aliments non consommés avant le repas du matin.
- Élever les géniteurs individuellement pendant 30 jours dans une salinité de 20 ppt.
- Enlevez les matières fécales et échangez 10% de l’eau de mer (20 ppt) par jour.
- Dissection
- Nettoyez un plateau de dissection, des ciseaux et des pinces avec de l’éthanol à 70 %.
- Anesthésiez les femelles individuellement avec la méthode d’anesthésie par immersion 2-PE.
- Choisir au hasard les femelles nouvellement matures (après la mue des femelles prématurées) qui n’ont pas subi d’ablation du pédoncule oculaire pour confirmer leurs stades gonadiques.
- Sacrifiez individuellement toutes les femelles expérimentales ablées par la tige oculaire et identifiez les stades de maturation des gonades. Détruisez les ganglions thoraciques du crabe à l’aide d’un poinçon stérile pointu. Enlevez d’abord la carapace supérieure, puis l’hépatopancréas pour rendre l’ovaire visible. Observez la couleur des ovaires et identifiez le stade de maturation ovarienne (Figure 2).
- Identification des stades de maturation ovarienne
- Observez la couleur des ovaires à l’œil nu ou au stéréomicroscope.
- Identifier les stades de maturation ovarienne en fonction de la coloration30 : l’immature (stade-1) présente une couleur blanche translucide ou crème ; La maturation précoce (stade 2) montre une couleur jaunâtre pâle à claire; iii) la prématuration (stade 3) présente une couleur jaune à orange clair; et (iv) la maturité complète (stade 4) montre une couleur orange foncé à rougeâtre.
Résultats
Maturation des gonades
Des tissus ovariens blanc crème (ovaires immatures, stade 1) ont été trouvés chez 100 % des femelles disséquées (n = 6) avant d’effectuer l’ablation du pédoncule oculaire (Figure 2). Le taux de maturation des gonades des crabes femelles ablées par le pédoncule oculaire (n = 63; 31 femelles avec la technique de cautérisation et 32 femelles avec la technique chirurgicale) était plus élevé que chez les crabes femelles qui n’ont pas s...
Discussion
Ce protocole a été développé pour l’ablation du pédoncule oculaire du crabe de vase, Scylla spp., et peut être appliqué comme méthode efficace pour induire la maturation des gonades. Ce protocole peut être facilement reproduit pour la maturation ovarienne commerciale des crabes de vase et peut être mis en œuvre pour réduire la période de latence (temps d’un frai à l’autre) dans la production de graines de crabe de vase.
L’ablation oculaire des crustacés (c.-à-d...
Déclarations de divulgation
Aucun des auteurs n’a de conflit d’intérêts.
Remerciements
Cette étude a été soutenue par le ministère de l’Éducation de Malaisie dans le cadre du programme Higher Institution Centre of Excellence (HICoE), Malaisie, accrédité auprès de l’Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu (Vot No. 63933 & Vot No. 56048). Nous reconnaissons le soutien de l’Universiti Malaysia Terengganu et de Sayap Jaya Sdn. Bhd. via la subvention de recherche en partenariat privé (vot. n ° 55377). Un poste de boursier académique adjoint de l’Universiti Sains Malaysia à Khor Waiho et Hanafiah Fazhan est également reconnu.
matériels
| Name | Company | Catalog Number | Comments |
| Aeration tube | Ming Yu Three | N/A | aquarium and pet shop |
| Airstone | Ming Yu Three | N/A | aquarium and pet shop |
| Autoclave machine | HIRAYAMA MANUFACTURING CORPORATION | N/A | MADE IN JAPAN |
| Bleaching powder (Hi-Chlon 70%) | Nippon Soda Co.Ltd,Japan | N/A | N/A |
| Blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Circular tank (32L) | BEST PLASTIC INDUSTRY SDN. BHD. | N/A | N/A |
| Cotton hand gloves (thick) | MR D.I.Y. Group Berhad | N/A | N/A |
| Cotton towel | MR D.I.Y. Group Berhad | N/A | N/A |
| Digital thermometer | Hanna Instrument | HI9814 | Hanna Instruments GroLine Hydroponics Waterproof pH / EC / TDS / Temp. Portable Meter HI9814 |
| Digital Vernier Caliper | INSIZE Co., Ltd. | N/A | |
| Dissecting tray | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Dropper bottle/Plastic Pipettes Dropper | Shopee Malaysia | N/A | N/A |
| Ethanol 70% | Thermo Scientific Chemicals | 033361.M1 | Diluted to 70% using double distilled water |
| Fiberglass tank (1 ton) | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Fine sand | N/A | N/A | collected from Sea beach of Universiti Malaysia Terengganu |
| First Aid Kits | Watsons Malaysia | N/A | N/A |
| Flat head nickel steel metal rod (Screw driver) | MR D.I.Y. Group Berhad | N/A | N/A |
| Formaldehyde | Thermo Scientific Chemicals | 119690010 | |
| Gas cylinder (butane gas) for blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Gas lighter gun (long head) | MR D.I.Y. Group Berhad | N/A | N/A |
| Glass beaker (100 mL)) | Corning Life Sciences | 1000-100 | |
| Ice bag | Watsons Malaysia | N/A | N/A |
| Perforated plastic baskets | Eco-Shop Marketing Sdn. Bhd. | N/A | N/A |
| PVC pipe 15mm | Bina Plastic Industries Sdn Bhd (HQ) | N/A | N/A |
| Refractometer | ATAGO CO.,LTD. | ||
| Refrigerator | Sharp Corporation Japan | N/A | Chest Freezer SHARP 110L - SJC 118 |
| Scoop net | MR D.I.Y. Group Berhad | N/A | |
| Seawater | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Siphoning pipe | MR D.I.Y. Group Berhad | N/A | N/A |
| Spray bottle | Mr. DIY Sdn Bhd | N/A | N/A |
| Stainless surgical forceps | N/A | N/A | N/A |
| Stainless surgical scissors | N/A | N/A | N/A |
| Submersible water pump | AS | N/A | model: Astro 4000 |
| Tincture of iodine solution (Povidone Iodine) | Farmasi Fajr Sdn Bhd | N/A | N/A |
| Tissue paper | N/A | N/A | |
| Transparent plastic aquarium | Ming Yu Three | N/A | aquarium and pet shop |
| Waterproof table | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
Références
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H., et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K., et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y., et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production. Aquaculture Reports. 18, 100432 (2020).
- Waiho, K., et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R., et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T., et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S., et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G., et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I., et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N., et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N., et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C., et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H. -. Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q., et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A., et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8, (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K., et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus. Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S. -. K., Lee, S. -. G., Lee, J. -. M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity. Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X., et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C., et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C., et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 130 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H., et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Erratum
Formal Correction: Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs
Posted by JoVE Editors on 5/26/2023. Citeable Link.
An erratum was issued for: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs. The Introduction, Protocol, Discussion and References were updated.
The forth sentence in the third paragraph of the Introduction has been updated from:
The eyestalk ablation protocol in this work minimizes stress by using fully sedated crabs and minimizes physical injury to personnel from crab bites.
to:
The eyestalk ablation protocol in this work minimizes stress by using fully anesthetized crabs and minimizes physical injury to personnel from crab bites.
The start of the Protocol has been updated from:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia. The sacrifice of the experimental samples was done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Sexually pre-mature female mud crabs (orange mud crab S. olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
to:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia and was approved by the Universiti Malaysia Terengganu's Research Ethics Committee (Animal ethics approval number: UMT/JKEPHMK/2023/96). The sacrifice of the experimental samples was done according to the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Sexually pre-mature female mud crabs (orange mud crab Scylla olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
Section 4 of the Protocol has been updated from:
4. Cold-shock anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for cold shock anesthesia.
- Prepare 2 L of 4 °C to 1 °C seawater (20 ppt) in a transparent plastic aquarium. Maintain the temperature using (−20 °C) ice bags for cold shock anesthesia.
NOTE: Check the temperature with a digital thermometer. - Immerse the crab in the 4 °C seawater until sedated (about 3−5 min).
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement. The legs and chelipeds joints will still show minor movements when touched with forceps.
to:
4. Anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for anesthesia.
- After 5 min of acclimatization period, add 2-phenoxyethanol (2-PE) at 2 mL/L into each aquarium and allow 15 min of anesthesia treatment.
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement.
Section 5 of the Protocol has been updated from:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized (sedated) crab with a wet cotton towel.
NOTE: Cover all the tentacles of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
- After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Prepare the tincture of iodine solution in a dropper bottle.
NOTE: Tincture of iodine (iodine tincture or weak iodine solution) is made up of 2%-7% elemental iodine and potassium iodide, or sodium iodide, dissolved in ethanol and water. - Wear thick cotton gloves.
- Hold the sedated crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
- Apply two to three drops of iodine tincture to the wounded part of the eyestalk immediately after cutting it off.
NOTE: Tincture of iodine is used for healing and to prevent infection.
to:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle and keep it away from any fire-related sources, such as blow torch and red hot screwdriver. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized crab with a wet cotton towel.
NOTE: Cover the antennae of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color. Be careful when conducting this step to avoid damage to adjacent structures.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
NOTE: Only perform this step at least waiting for 5 min after the eyestalk ablation procedure to ensure the forceps are cooled down before disinfection using 70% ethanol to prevent potential fire hazards. - After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Wear thick cotton gloves.
- Hold the anesthetized crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
Step 7.2.2 of the Protocol has been updated from:
Sedate the females individually with the cold shock anesthesia method.
to:
Anesthetize the females individually with the 2-PE immersion anesthesia method.
The Discussion has been updated from:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,40,41. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,42. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,42,43. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,44. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique requires an additional step of disinfection using iodine. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,45,46.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. The application of a tincture of iodine can prevent infection of the wounded part. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,47. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,48,49.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,50,51. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,52. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
to:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Anesthesia via immersion in 2-phenoxyethanol was used as it is comparable to the use of tricaine methanesulfonate (MS-222) in arthopods but cheaper and does not require the use of additional buffer40. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,41,42. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,43. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,43,44. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,45. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique takes time for the wound to heal and this would allow for chance of infection. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,46,47.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,48. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,49,50. In addition, future research on pain assessment following eyestalk ablation on mud crabs is recommended to highlight the change in behaviours associated with pain and stress, as evident in freshwater prawn Macrobrachium americanum51.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,52,53. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,53. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
The References have been updated from:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
to:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon