A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs
In This Article
Erratum Notice
Summary
Two eyestalk ablation protocols (i.e., cauterization and surgery approaches) were performed on anesthetized female crabs. The eyestalk ablation of mud crabs hastened the ripening of ovaries without decreasing the survival rate.
Abstract
Mud crabs (Scylla spp.) are commercially important crustacean species that can be found throughout the Indo-West Pacific region. During culture, the induction of ovarian maturation is important to meet the consumer demand for mature mud crabs and hasten seed production. Eyestalk ablation is an effective tool to enhance ovarian maturation in mud crabs. However, there is no standard protocol for the eyestalk ablation of mud crabs. In this study, two eyestalk ablation techniques are described: cauterization (the use of hot metal to ablate the eyestalk of an anesthetized crab) and surgery (the removal of the eyestalk using surgical scissors). Before eyestalk ablation, sexually mature females (CW > 86 mm) were anesthetized using an ice bag (−20 °C) with seawater. When the water temperature reached 4 °C, the ice bag was removed from the water. Flowing seawater (ambient temperature: 28 °C) was used for recovery from the anesthesia immediately after eyestalk ablation. Mortality did not occur during or after the process of eyestalk ablation. The eyestalk ablation protocol presented here accelerated the ovarian maturation of the mud crabs.
Introduction
All four mud crab species belonging to the genus Scylla are commercially important crustacean species in aquaculture1,2. The growth of crustaceans, including mud crabs, and their transformation from the pre-mature (sub-adult or pubertal) phase to the sexually mature (adult) phase occur through a molting process that involves the periodic shedding of older and smaller exoskeletons. Carapace width (CW), chelipeds, and abdominal flap morphologies are widely used to determine the sexual maturity of Scylla spp.3,4,5. The process of molting is regulated by the action of various hormones and requires a huge amount of energy6. In addition to the normal molting process, the loss of limbs, either voluntarily or induced by external factors, expedites the molting of crabs without affecting their survival rate7,8,9. Therefore, limb autotomy is commonly used for molt induction in the soft-shell mud crab farming industry7,9.
Unilateral or bilateral eyestalk ablation is mostly popular in freshwater prawn and marine shrimp for gonad maturation and seed production10,11,12,13. Common eyestalk ablation techniques in crustaceans include the following: (i) ligation at the base of the eyestalk using a string14,15; (ii) cauterization of the eyestalk using hot forceps or electrocautery devices16; (iii) removal or direct pinching of the eyestalk to leave an open wound12; and (iv) removal of the eyestalk contents through incision after slicing the distal portion of the eye with a razor17. The eyestalk X-organs are important endocrine organs in crustaceans as they regulate crustacean hyperglycemic hormones (CHH), molt-inhibiting hormones (MIH), and vitellogenesis-inhibiting hormones (VIH)6,18,19,20,21,22. Eyestalk X-organs (or the sinus gland complex) synthesize and release gonad-inhibiting hormones (GIH), also known as vitellogenesis-inhibiting hormones (VIH), belonging to the neuropeptide hormone family6. Unilateral or bilateral eyestalk ablation reduces GIH synthesis, resulting in the dominance of stimulating hormones (i.e., gonad stimulating hormones, GSH) and the acceleration of the ovarian maturation process in crustaceans23,24,25,26. Without the influence of GIH after eyestalk ablation, female crustaceans devote their energy to ovary development27. It has been found that unilateral eyestalk ablation is sufficient for the induction of ovarian maturation in crustaceans11 and that the ablated eyestalk of shrimps and crabs can regenerate after several moltings28. There are four ovarian development stages recorded in Scylla spp.: i) immature (stage-1), ii) early maturing (stage-2), iii) pre-maturing (stage-3), and iv) fully mature (stage-4)29,30. The immature ovarian stage is found in immature females. After pubertal molting and mating, the immature ovary starts developing and finally matures (stage-4) before spawning31.
An eyestalk ablation protocol is essential for mud crab broodstock development and seed production. In the global food market, mature mud crabs with fully mature ovaries (stage-4) rather than crabs with higher muscle content are preferred by consumers and, thus, have a higher commercial value, even higher than large males. There is no complete protocol for the eyestalk ablation of mud crabs. The eyestalk ablation protocol in this work minimizes stress by using fully anesthetized crabs and minimizes physical injury to personnel from crab bites. This protocol is easy and cost-effective. Here, we present a protocol for the eyestalk ablation of Scylla spp. that can induce the maturation of the gonad. Two techniques of eyestalk ablation (cauterization and surgery) were tested and their efficiencies compared based on the gonadal development rate of female mud crabs.
Protocol
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia. The sacrifice of the experimental samples was done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Sexually pre-mature female mud crabs (orange mud crab S. olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
1. Sample collection and disinfection
- Collect healthy, active, and pre-mature female mud crabs (Figure 1).
NOTE: Pre-mature female crabs have triangular and light-colored abdomen flaps along with a CW range of 80−85 mm. - Wash the crabs with chlorinated tap water (freshwater) to remove debris and osmophilic parasites.
- Soak the crabs in 150 ppm formaldehyde with 20 ppt salinity for 30 min.
- Maintain continuous and gentle aeration with airstones during the formaldehyde treatment. The aeration source can be from either a central aeration line or an aquarium aerator pump.
- Wash the crabs with flowing seawater to remove any residual formaldehyde.
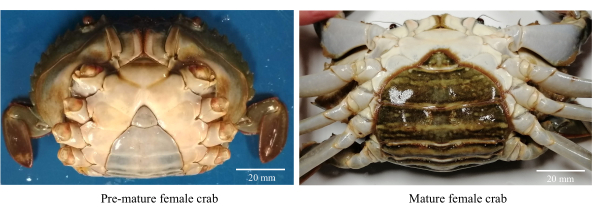
Figure 1: Abdominal morphology of female mud crabs used to identify the sexual maturation stages. Please click here to view a larger version of this figure.
2. Acclimatization
- Transfer each disinfected female into a separate 32 L circular tank.
- Rear the females for 3 days in 20 ppt salinity and continue feeding twice per day (morning 09:00 am and evening 20:00 pm) with chopped marine fish at about 4%−5% of the crab's body weight.
- Remove excess and uneaten feed by siphoning before the morning feeding.
- Exchange 10% of the crab rearing seawater (20 ppt) daily.
3. Induced molting for sexual maturity
- Cut all the legs except the swimming legs using sterilized scissors.
- Catch the crab with a scoop net, and hold the crab carefully. Cut both chelipeds first and then the walking legs at the second joint using scissors. The crab will autotomize the damaged appendages automatically. Anaesthesia is not required for limb autotomy.
- Wash the crab in freshwater immediately after limb autotomy.
- Individually transfer the limb-autotomized crabs into perforated plastic baskets (28 cm L x 22 cm W x 7 cm H), and place them in a fiberglass tank (305 cm L x 120 cm W x 60 cm H).
NOTE: Two baskets can be tied and clipped together. The top basket is used as a cover so that the crab cannot escape from the basket. - Use a recirculating aquaculture system (RAS) with 20 ppt salinity and a water depth of at least 10 cm to ensure that the whole plastic basket is submerged.
- Continue feeding the limb-autotomized female crab with chopped marine fish twice per day at 5%−7% of the crab's body weight.
- Rear the crabs until sexually mature through molting (35 days).
NOTE: Induced molting can be skipped for commercial ovarian maturation and seed production with wild mature female mud crabs. Harvested mature females from the wild must be acclimatized and directly subjected to cold-shock anesthesia and subsequent eyestalk ablation.
4. Anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for anesthesia.
- After 5 min of acclimatization period, add 2-phenoxyethanol (2-PE) at 2 mL/L into each aquarium and allow 15 min of anesthesia treatment.
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement.
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle and keep it away from any fire-related sources, such as blow torch and red hot screwdriver. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized crab with a wet cotton towel.
NOTE: Cover the antennae of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color. Be careful when conducting this step to avoid damage to adjacent structures.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
NOTE: Only perform this step at least waiting for 5 min after the eyestalk ablation procedure to ensure the forceps are cooled down before disinfection using 70% ethanol to prevent potential fire hazards. - After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Wear thick cotton gloves.
- Hold the anesthetized crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
6. Post-anesthesia care
- Prepare 20 ppt filtered seawater, and keep in an overhead tank with continuous aeration.
- Connect a flexible pipe with the overhead tank for gravitational water flow.
- Immediately after eyestalk ablation, place the crab into the basket, and subject the crab to flowing seawater (ambient water temperature: 28 °C) from the overhead tank.
- Keep the seawater flowing, and monitor the crab until it can move spontaneously, which indicates recovery from the anesthesia.
NOTE: Seawater can be prepared in a ground tank, and a submersible water pump can be used for the water flow. - Keep the crabs individually in 20 ppt seawater with aeration in an aquarium for 30 min for further observation.
NOTE: The recovered crabs will be cultured individually in the subsequent broodstock culture process.
7. Observation of ovarian maturation
- Broodstock rearing
- Transfer the mature crabs to individual 32 L circular tanks.
- Continue feeding with chopped marine fish (frozen at −20 °C) twice per day (morning 09:00 am and evening 20:00 pm), and remove uneaten feed before the morning feeding.
- Rear the broodstock individually for 30 days in 20 ppt salinity.
- Remove feces, and exchange 10% of the seawater (20 ppt) daily.
- Dissection
- Clean a dissecting tray, scissors, and forceps with 70% ethanol.
- Anesthetize the females individually with the 2-PE immersion anesthesia method.
- Randomly select newly mature females (after the molting of pre-mature females) that have not gone through eyestalk ablation to confirm their gonadal stages.
- Sacrifice all the eyestalk-ablated experimental females individually, and identify the gonad maturation stages. Destroy the thoracic ganglia of the crab using a sharp sterile awl. Remove the top carapace first and then the hepatopancreas to make the ovary visible. Observe the ovary color, and identify the ovarian maturation stage (Figure 2).
- Ovarian maturation stages identification
- Observe the ovary color with the naked eye or under a stereomicroscope.
- Identify the ovarian maturation stages based on coloration30: the immature (stage-1) shows a translucent or creamy white color; the early maturing (stage-2) shows a pale to light yellowish color; (iii) the pre-maturing (stage-3) shows a yellow to light orange color; and (iv) the fully matured (stage-4) shows a dark orange to reddish color.
Results
Gonad maturation
Creamy white ovarian tissues (immature ovaries, stage-1) were found in 100% of the dissected females (n = 6) before performing the eyestalk ablation (Figure 2). The gonad maturation rate of the eyestalk-ablated female crabs (n = 63; 31 females with the cauterization technique and 32 females with the surgery technique) was higher compared to female crabs that were not subjected to eyestalk ablation (n = 31) after 30 days of individual rearing (
Discussion
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-o...
Disclosures
None of the authors have any conflicts of interest.
Acknowledgements
This study was supported by the Ministry of Education, Malaysia, under the Higher Institution Centre of Excellence (HICoE) program, Malaysia, accredited to the Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu (Vot No. 63933 & Vot No. 56048). We acknowledge the support of Universiti Malaysia Terengganu and Sayap Jaya Sdn. Bhd. via the Private Partnership Research Grant (Vot. No. 55377). An adjunct Academic Fellow position from Universiti Sains Malaysia to Khor Waiho and Hanafiah Fazhan is also acknowledged.
Materials
| Name | Company | Catalog Number | Comments |
| Aeration tube | Ming Yu Three | N/A | aquarium and pet shop |
| Airstone | Ming Yu Three | N/A | aquarium and pet shop |
| Autoclave machine | HIRAYAMA MANUFACTURING CORPORATION | N/A | MADE IN JAPAN |
| Bleaching powder (Hi-Chlon 70%) | Nippon Soda Co.Ltd,Japan | N/A | N/A |
| Blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Circular tank (32L) | BEST PLASTIC INDUSTRY SDN. BHD. | N/A | N/A |
| Cotton hand gloves (thick) | MR D.I.Y. Group Berhad | N/A | N/A |
| Cotton towel | MR D.I.Y. Group Berhad | N/A | N/A |
| Digital thermometer | Hanna Instrument | HI9814 | Hanna Instruments GroLine Hydroponics Waterproof pH / EC / TDS / Temp. Portable Meter HI9814 |
| Digital Vernier Caliper | INSIZE Co., Ltd. | N/A | |
| Dissecting tray | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Dropper bottle/Plastic Pipettes Dropper | Shopee Malaysia | N/A | N/A |
| Ethanol 70% | Thermo Scientific Chemicals | 033361.M1 | Diluted to 70% using double distilled water |
| Fiberglass tank (1 ton) | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Fine sand | N/A | N/A | collected from Sea beach of Universiti Malaysia Terengganu |
| First Aid Kits | Watsons Malaysia | N/A | N/A |
| Flat head nickel steel metal rod (Screw driver) | MR D.I.Y. Group Berhad | N/A | N/A |
| Formaldehyde | Thermo Scientific Chemicals | 119690010 | |
| Gas cylinder (butane gas) for blow torch | MR D.I.Y. Group Berhad | N/A | N/A |
| Gas lighter gun (long head) | MR D.I.Y. Group Berhad | N/A | N/A |
| Glass beaker (100 mL)) | Corning Life Sciences | 1000-100 | |
| Ice bag | Watsons Malaysia | N/A | N/A |
| Perforated plastic baskets | Eco-Shop Marketing Sdn. Bhd. | N/A | N/A |
| PVC pipe 15mm | Bina Plastic Industries Sdn Bhd (HQ) | N/A | N/A |
| Refractometer | ATAGO CO.,LTD. | ||
| Refrigerator | Sharp Corporation Japan | N/A | Chest Freezer SHARP 110L - SJC 118 |
| Scoop net | MR D.I.Y. Group Berhad | N/A | |
| Seawater | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
| Siphoning pipe | MR D.I.Y. Group Berhad | N/A | N/A |
| Spray bottle | Mr. DIY Sdn Bhd | N/A | N/A |
| Stainless surgical forceps | N/A | N/A | N/A |
| Stainless surgical scissors | N/A | N/A | N/A |
| Submersible water pump | AS | N/A | model: Astro 4000 |
| Tincture of iodine solution (Povidone Iodine) | Farmasi Fajr Sdn Bhd | N/A | N/A |
| Tissue paper | N/A | N/A | |
| Transparent plastic aquarium | Ming Yu Three | N/A | aquarium and pet shop |
| Waterproof table | Hatcheri AKUATROP | N/A | Research Center of Universiti Malaysia Terengganu |
References
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H., et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K., et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y., et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production. Aquaculture Reports. 18, 100432 (2020).
- Waiho, K., et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R., et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T., et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S., et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G., et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I., et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N., et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N., et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C., et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H. -. Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q., et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A., et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8, (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K., et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus. Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S. -. K., Lee, S. -. G., Lee, J. -. M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity. Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X., et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C., et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C., et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 130 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H., et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Erratum
Formal Correction: Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs
Posted by JoVE Editors on 5/26/2023. Citeable Link.
An erratum was issued for: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs. The Introduction, Protocol, Discussion and References were updated.
The forth sentence in the third paragraph of the Introduction has been updated from:
The eyestalk ablation protocol in this work minimizes stress by using fully sedated crabs and minimizes physical injury to personnel from crab bites.
to:
The eyestalk ablation protocol in this work minimizes stress by using fully anesthetized crabs and minimizes physical injury to personnel from crab bites.
The start of the Protocol has been updated from:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia. The sacrifice of the experimental samples was done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Sexually pre-mature female mud crabs (orange mud crab S. olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
to:
This protocol follows the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes outlined by the Laboratory Animal Science Association of Malaysia and was approved by the Universiti Malaysia Terengganu's Research Ethics Committee (Animal ethics approval number: UMT/JKEPHMK/2023/96). The sacrifice of the experimental samples was done according to the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Sexually pre-mature female mud crabs (orange mud crab Scylla olivacea) were collected from the local market (5°66′62′′N, 102°72′33′′E) at the Setiu Wetlands in Malaysia. The mud crab species was identified based on morphological characteristics1.
Section 4 of the Protocol has been updated from:
4. Cold-shock anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for cold shock anesthesia.
- Prepare 2 L of 4 °C to 1 °C seawater (20 ppt) in a transparent plastic aquarium. Maintain the temperature using (−20 °C) ice bags for cold shock anesthesia.
NOTE: Check the temperature with a digital thermometer. - Immerse the crab in the 4 °C seawater until sedated (about 3−5 min).
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement. The legs and chelipeds joints will still show minor movements when touched with forceps.
to:
4. Anesthesia
- Select sexually mature females with a dark-colored oval-shaped abdominal flap with a CW >86 mm (Figure 1).
- Catch the crabs with a scoop net, and keep them individually in small aquariums for anesthesia.
- After 5 min of acclimatization period, add 2-phenoxyethanol (2-PE) at 2 mL/L into each aquarium and allow 15 min of anesthesia treatment.
- Ensure the crabs are fully anesthetized by the lack of spontaneous movement.
Section 5 of the Protocol has been updated from:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized (sedated) crab with a wet cotton towel.
NOTE: Cover all the tentacles of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
- After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Prepare the tincture of iodine solution in a dropper bottle.
NOTE: Tincture of iodine (iodine tincture or weak iodine solution) is made up of 2%-7% elemental iodine and potassium iodide, or sodium iodide, dissolved in ethanol and water. - Wear thick cotton gloves.
- Hold the sedated crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
- Apply two to three drops of iodine tincture to the wounded part of the eyestalk immediately after cutting it off.
NOTE: Tincture of iodine is used for healing and to prevent infection.
to:
5. Eyestalk ablation
- Cauterization technique
- Perform all procedures on top of a table and in an open area.
- Take a flat head nickel-steel metal rod (e.g., a screwdriver) with a wooden or plastic handle, and cover the handle with a wet cotton towel.
- Sterilize two stainless surgical forceps in an autoclave.
- Prepare 70% ethanol in a spray bottle and keep it away from any fire-related sources, such as blow torch and red hot screwdriver. Have tissue paper ready for use.
NOTE: Ethanol is highly flammable. Maintain a safe distance from fire sources. - Connect a blowtorch to a gas cylinder (butane) securely.
CAUTION: Follow the instructions on the blowtorch and gas cylinder. Make sure that the blowtorch is switched off when connecting with the gas cylinder. Read and follow all the fire safety precautions mentioned on the gas cylinder. - Wear thick cotton gloves to avoid injury from hot objects.
- Subject the tip of the metal rod to the fire of the blowtorch until the metal rod is bright red.
- Cover the anesthetized crab with a wet cotton towel.
NOTE: Cover the antennae of the crab to avoid unnecessary damage. - Hold one eye of the crab with sterilized forceps.
NOTE: Sterilize the forceps in an autoclave for first-time use, and disinfect using 70% ethanol for subsequent use on other crabs. - Hold the red-hot metal flat tip onto the eye of the crab and press slightly for about 10−15 s until the eyestalk turns an orange or reddish-orange color. Be careful when conducting this step to avoid damage to adjacent structures.
NOTE: Two people are needed to execute eyestalk ablation following the cauterization method: one to hold the crab and another to perform the ablation procedure. - Disinfect the forceps with 70% ethanol spray to ensure no cross-contamination between crabs.
NOTE: Only perform this step at least waiting for 5 min after the eyestalk ablation procedure to ensure the forceps are cooled down before disinfection using 70% ethanol to prevent potential fire hazards. - After performing the eyestalk ablation on all crabs, dip the hot nickel steel metal rod (screwdriver) into tap water.
- Disinfect the towel before reuse. Multiple towels can be used to save time.
NOTE: Wash the towel with tap water, and dip it into 30 ppm chlorinated water for 5 min. Then, wash the towel with tap water again, and dip it in a 1 g/L sodium thiosulphate solution. - Keep the blowtorch in a safe place after turning it off, and wait until it returns to environmental temperature (about 30 min) before disconnecting.
- Surgery technique
- Perform the procedure in a well-ventilated area.
- Sterilize two surgical scissors and forceps in an autoclave.
- Pour 50 mL of 70% ethanol into a 100 mL glass beaker.
- Wear thick cotton gloves.
- Hold the anesthetized crab, and cover it with a wet cotton towel.
- Hold one eye of the crab with sterilized forceps.
- Swiftly cut off the eyestalk using sterilized surgical scissors.
NOTE: Hemolymph may be lost from the wounded part of the crab. - Dip the scissors and forceps in 70% ethanol after every use, and dry them using tissue paper before reuse.
Step 7.2.2 of the Protocol has been updated from:
Sedate the females individually with the cold shock anesthesia method.
to:
Anesthetize the females individually with the 2-PE immersion anesthesia method.
The Discussion has been updated from:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,40,41. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,42. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,42,43. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,44. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique requires an additional step of disinfection using iodine. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,45,46.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. The application of a tincture of iodine can prevent infection of the wounded part. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,47. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,48,49.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,50,51. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,52. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
to:
This protocol was developed for the eyestalk ablation of the mud crab, Scylla spp., and can be applied as an efficient method to induce gonad maturation. This protocol can be easily replicated for the commercial ovary maturation of mud crabs and can be implemented to reduce the latent period (time from one spawning to another) in mud crab seed production.
The eyestalk ablation of crustaceans (i.e., freshwater prawn, marine shrimp) is typically done to induce gonad maturation and out-of-season spawning11,12,13. Eyestalk ablation in brachyuran crabs has also been done to study molting25,32,33, hormonal regulation18, gonad maturation34, and induced breeding and reproductive performance35,36,37,38,39. Anesthesia via immersion in 2-phenoxyethanol was used as it is comparable to the use of tricaine methanesulfonate (MS-222) in arthopods but cheaper and does not require the use of additional buffer40. Unilateral or bilateral eyestalk ablation influences the physiology of the crustacean. Eyestalk ablation following the protocol stated in this study also influences the ovarian maturation rate of mud crabs. In the control treatment (without eyestalk ablation), 43.33% ± 5.77% of female crabs had an immature ovary (stage-1). However, in the same rearing period (30 days), eyestalk-ablated female crabs had pre-maturing ovaries (stage-3; 56.67% ± 11.55% and 53.33% ± 15.28% with the cauterization and surgery techniques, respectively), which shows that eyestalk ablation can increase the gonad maturation of mud crabs. Previous studies have also reported that the ovarian development of intact crabs (without eyestalk ablation) is slower than that of eyestalk-ablated crabs25,31. Due to the slower gonadal development in intact crustaceans, eyestalk ablation is widely done in commercial prawn and shrimp hatcheries. In this protocol, the eyestalk-ablated female crabs achieved higher percentages of ovarian maturation compared to the female crabs without the eyestalk ablation treatment (Figure 3).
The gonad maturation of the mud crab is regulated by hormones21,41,42. The eyestalk contains important endocrine glands (i.e., the X-organ-sinus gland complex) that play vital roles in the gonadal maturation process of mud crabs18,21. Unilateral eyestalk ablation, either by cauterization or surgery, damages one of the major endocrine glands that is involved in the synthesis and release of inhibiting hormones (e.g., VIH), thereby resulting in a higher level of gonad-stimulating hormones (i.e., VSH).
The ovarian maturation stages of Scylla spp. can be differentiated by observing the ovarian tissue coloration with the naked eye29,30,43. Translucent or creamy white ovarian tissues are indications of immature ovaries29,30,43,44. In this study, immature ovaries (stage-1) were still found in the group of female crabs without eyestalk ablation due to the slower ovarian maturation process. However, the crabs in the eyestalk-ablated groups (both by the cauterization and surgery techniques) mostly showed pre-maturing ovaries (stage-3), with some individuals exhibiting fully matured ovaries (stage-4). Therefore, the protocol of eyestalk ablation described here can be used to increase ovarian maturation in female mud crabs. This protocol can also be applied directly to wild-collected mature female mud crabs to hasten their seed production. To evaluate the effectiveness of cauterization and surgery methods on mud crab gonad maturation and to ensure the accurate estimation of molting duration, sexually pre-mature crabs were used. After the (induced) molting of sexually pre-mature female crabs, we noticed that their ovaries were still in the immature or early developing stages29,45. After 30 days of rearing the newly mature female crabs (either eyestalk-ablated or without eyestalk ablation), the ovarian development stages (stage-1 to stage-4) were determined by the color of the ovarian tissues. This protocol encourages the use of the cauterization technique to perform eyestalk ablation in mud crabs to avoid any hemolymph loss and prevent infection at the ablated sites. Cauterization immediately seals the wound, whereas the surgery technique takes time for the wound to heal and this would allow for chance of infection. For commercial purposes, larger mature crabs, preferably at a later stage of ovarian maturation, should be selected for eyestalk ablation to shorten the time to reach the fully matured ovary stage for subsequent commerce or brood stock culture. In addition to eyestalk ablation, individual rearing with sand substrate and sufficient feeding, preferably with live feed, can increase the gonad maturation rate of mud crabs in captivity30,35,46,47.
Crustacean blood is called hemolymph and can be lost during eyestalk ablation. An excessive loss of hemolymph may lead to the death of eyestalk-ablated crabs, especially when performing surgery to remove the eyestalk. The hemolymph can coagulate in the wounded part to prevent loss. However, in comparison to the surgery technique, the cauterization technique seals the wounded part immediately, thereby preventing the loss of hemolymph and possible infection.
Mud crab mortality after unilateral eyestalk ablation with either cauterization or surgery was not found within the first 7 days. Thus, eyestalk ablation can be done with a higher survival rate. Unilateral eyestalk ablation does not hamper the survival rate of the crab33.
Stress during crab handling and eyestalk ablation may contribute to crab mortality. Proper anesthesia is needed to minimize handling stress during eyestalk ablation. In crustacean eyestalk ablation, chemical anesthetics (i.e., xylocaine, lidocaine) are used at the base of the eyestalk before eyestalk ablation14,15,17,48. However, due to the aggressive nature and large size of mud crabs, the use of anesthesia only at the base of the eyestalk is not sufficient and might result in additional stress to the animals during the injection. On the other hand, anesthesia by subjecting them to a lower water temperature is more economical and safer. The use of cold water for anesthesia in mud crabs is common and has been used in other studies due to its efficiency, simplicity, and minimal impact on recovery and survival37,49,50. In addition, future research on pain assessment following eyestalk ablation on mud crabs is recommended to highlight the change in behaviours associated with pain and stress, as evident in freshwater prawn Macrobrachium americanum51.
Although eyestalk ablation using both cauterization and surgery methods has a minimal effect on crab survival and enhances ovarian maturation, performing eyestalk ablation requires professional mastery of the techniques. The timing between the steps is critical as any delay between protocols adds additional stress for the crabs. Unlike the surgery technique, the cauterization technique is dangerous because it involves the use of flammable equipment (i.e., a blow torch and butane gas). Thus, extra caution is needed when performing the cauterization technique.
Crabs are cannibalistic in nature, and they are known to prey on others that have just completed their molt and are still in their soft-shell conditions7,52,53. Thus, rearing the crabs individually can avoid unnecessary mortality due to cannibalism. The use of individual rearing in mud crab culture is commonly practiced, both in high-density culture and pond culture, for fattening and soft-shell crab farming purposes8,53. This protocol also utilized individual rearing and maintenance. During the transportation of the crabs for rearing or commerce, the crab chelipeds are tied up securely (or even autotomized) to prevent fighting, unnecessary injury, and limb loss34.
The described protocol for eyestalk ablation should be performed with multiple persons. After completing the eyestalk ablation, non-disposable equipment (e.g., the aquarium, tray, towel, etc.) should be disinfected with 30 ppm chlorine. The crabs must be monitored at least twice per day. Any dead crabs, uneaten feed, ablated limbs, or molted crab shells should be swiftly disposed of (i.e., buried in soil with bleaching powder) to prevent any potential for disease spread.
The References have been updated from:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
to:
- Keenan, C. P., Davie, P. J. F., Mann, D. L. A revision of the genus Scylla de Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bulletin of Zoology. 46 (1), 217-245 (1998).
- Fazhan, H. et al. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ. 8, e8066 (2020).
- Ikhwanuddin, M., Bachok, Z., Hilmi, M. G., Azmie, G., Zakaria, M. Z. Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters, Malaysia. Journal of Sustainability Science and Management. 5 (2), 97-109 (2010).
- Ikhwanuddin, M., Bachok, Z., Mohd Faizal, W. W. Y., Azmie, G., Abol-Munafi, A. B. Size of maturity of mud crab Scylla olivacea (Herbst, 1796) from mangrove areas of Terengganu coastal waters. Journal of Sustainability Science and Management. 5 (2), 134-147 (2010).
- Waiho, K. et al. On types of sexual maturity in brachyurans, with special reference to size at the onset of sexual maturity. Journal of Shellfish Research. 36 (3), 807-839 (2017).
- Mykles, D. L., Chang, E. S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. General and Comparative Endocrinology. 294, 113493 (2020).
- Fujaya, Y. et al. Is limb autotomy really efficient compared to traditional rearing in soft-shell crab (Scylla olivacea) production? Aquaculture Reports. 18, 100432 (2020).
- Waiho, K. et al. Moult induction methods in soft-shell crab production. Aquaculture Research. 52 (9), 4026-4042 (2021).
- Rahman, M. R. et al. Evaluation of limb autotomy as a promising strategy to improve production performances of mud crab (Scylla olivacea) in the soft-shell farming system. Aquaculture Research. 51 (6), 2555-2572 (2020).
- Okumura, T. et al. Expression of vitellogenin and cortical rod proteins during induced ovarian development by eyestalk ablation in the kuruma prawn, Marsupenaeus japonicus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 143 (2), 246-253 (2006).
- Pervaiz, P. A., Jhon, S. M., Sikdar-bar, M. Studies on the effect of unilateral eyestalk ablation in maturation of gonads of a freshwater prawn Macrobrachium dayanum. World Journal of Zoology. 6 (2), 159-163 (2011).
- Primavera, J. H. Induced maturation and spawning in five-month-old Penaeus monodon Fabricius by eyestalk ablation. Aquaculture. 13 (4), 355-359 (1978).
- Shyne Anand, P. S. et al. Reproductive performance of wild brooders of Indian white shrimp, Penaeus indicus: Potential and challenges for selective breeding program. Journal of Coastal Research. 86 (sp1), 65 (2019).
- Diarte-Plata, G. et al. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Vargas-Téllez, I. et al. Impact of unilateral eyestalk ablation on Callinectes arcuatus (Ordway, 1863) under laboratory conditions: Behavioral evaluation. Latin American Journal of Aquatic Research. 49 (4), 576-594 (2021).
- Chu, K. H., Chow, W. K. Effects of unilateral versus bilateral eyestalk ablation on molting and growth of the shrimp, Penaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidea). Crustaceana. 62 (3), 225-233 (1992).
- Taylor, J. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. 233 (1-4), 173-179 (2004).
- Wang, M., Ye, H., Miao, L., Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture. 560, 738493 (2022).
- Nagaraju, G. P. C. Reproductive regulators in decapod crustaceans: an overview. Journal of Experimental Biology. 214 (1), 3-16 (2011).
- Kornthong, N. et al. Characterization of red pigment concentrating hormone (RPCH) in the female mud crab (Scylla olivacea) and the effect of 5-HT on its expression. General and Comparative Endocrinology. 185, 28-36 (2013).
- Kornthong, N. et al. Molecular characterization of a vitellogenesis-inhibiting hormone (VIH) in the mud crab (Scylla olivacea) and temporal changes in abundances of VIH mRNA transcripts during ovarian maturation and following neurotransmitter administration. Animal Reproduction Science. 208, 106122 (2019).
- Liu, C. et al. VIH from the mud crab is specifically expressed in the eyestalk and potentially regulated by transactivator of Sox9/Oct4/Oct1. General and Comparative Endocrinology. 255, 1-11 (2018).
- Chen, H.-Y., Kang, B. J., Sultana, Z., Wilder, M. N. Variation of protein kinase C-α expression in eyestalk removal-activated ovaries in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 237 (300), 110552 (2019).
- Rotllant, G., Nguyen, T. V., Aizen, J., Suwansa-ard, S., Ventura, T. Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia. 825 (1), 91-119 (2018).
- Supriya, N. T., Sudha, K., Krishnakumar, V., Anilkumar, G. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda). Chinese Journal of Oceanology and Limnology. 35 (3), 645-657 (2017).
- Wilder, M. N. Advances in the science of crustacean reproductive physiology and potential applications to new seed production technology. Journal of Coastal Research. 86 (sp1), 6-10 (2019).
- Arcos, G. F., Ibarra, A. M., Vazquez-Boucard, C., Palacios, E., Racotta, I. S. Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquaculture Research. 34 (9), 749-755 (2003).
- Desai, U. M., Achuthankutty, C. T. Complete regeneration of ablated eyestalk in penaeid prawn, Penaeus monodon. Current Science. 79 (11), 1602-1603 (2000).
- Wu, Q. et al. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture. 515, 734560 (2020).
- Ghazali, A., Azra, M. N., Noordin, N. M., Abol-Munafi, A. B., Ikhwanuddin, M. Ovarian morphological development and fatty acids profile of mud crab (Scylla olivacea) fed with various diets. Aquaculture. 468 (Part 1), 45-52 (2017).
- Farhadi, A. et al. The regulatory mechanism of sexual development in decapod crustaceans. Frontiers in Marine Science. 8 (2021).
- Sukardi, P., Prayogo, N. A., Harisam, T., Sudaryono, A. Effect of eyestalk-ablation and differences salinity in rearing pond on molting speed of Scylla serrata. AIP Conference Proceedings. 2094, 020029 (2019).
- Stella, V. S., López Greco, L. S., Rodríguez, E. M. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). Journal of Crustacean Biology. 20 (2), 239-244 (2000).
- Jang, I. K. et al. The effects of manipulating water temperature, photoperiod, and eyestalk ablation on gonad maturation of the swimming crab, Portunus trituberculatus.Crustaceana. 83 (2), 129-141 (2010).
- Millamena, O. M., Quinitio, E. The effects of diets on reproductive performance of eyestalk ablated and intact mud crab Scylla serrata. Aquaculture. 181 (1-2), 81-90 (2000).
- Zeng, C. Induced out-of-season spawning of the mud crab, Scylla paramamosain (Estampador) and effects of temperature on embryo development. Aquaculture Research. 38 (14), 1478-1485 (2007).
- Rana, S. Eye stalk ablation of freshwater crab, Barytelphusa lugubris: An alternative approach of hormonal induced breeding. International Journal of Pure and Applied Zoology. 6 (3), 30-34 (2018).
- Yi, S.-K., Lee, S.-G., Lee, J.-M. Preliminary study of seed production of the Micronesian mud crab Scylla serrata (Crustacea: Portunidae) in Korea. Ocean and Polar Research. 31 (3), 257-264 (2009).
- Azra, M. N., Abol-Munafi, A. B., Ikhwanuddin, M. A review of broodstock improvement to brachyuran crab: Reproductive performance. International Journal of Aquaculture. 5 (38), 1-10 (2016).
- Archibald, K. E., Scott, G. N., Bailey, K. M., Harms, C. A. 2-phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). Journal of Zoo and Wildlife Medicine. 50 (1), 96-106 (2019).
- Muhd-Farouk, H., Abol-Munafi, A. B., Jasmani, S., Ikhwanuddin, M. Effect of steroid hormones 17α-hydroxyprogesterone and 17α-hydroxypregnenolone on ovary external morphology of orange mud crab, Scylla olivacea. Asian Journal of Cell Biology. 9 (1), 23-28 (2013).
- Muhd-Farouk, H., Jasmani, S., Ikhwanuddin, M. Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796). Aquaculture. 451, 78-86 (2016).
- Ghazali, A., Mat Noordin, N., Abol-Munafi, A. B., Azra, M. N., Ikhwanuddin, M. Ovarian maturation stages of wild and captive mud crab, Scylla olivacea fed with two diets. Sains Malaysiana. 46 (12), 2273-2280 (2017).
- Aaqillah-Amr, M. A., Hidir, A., Noordiyana, M. N., Ikhwanuddin, M. Morphological, biochemical and histological analysis of mud crab ovary and hepatopancreas at different stages of development. Animal Reproduction Science. 195, 274-283 (2018).
- Amin-Safwan, A., Muhd-Farouk, H., Mardhiyyah, M. P., Nadirah, M., Ikhwanuddin, M. Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity? Journal of King Saud University - Science. 31 (4), 827-835 (2019).
- Wu, X. et al. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture. 273 (4), 602-613 (2007).
- Azra, M. N., Ikhwanuddin, M. A review of maturation diets for mud crab genus Scylla broodstock: Present research, problems and future perspective. Saudi Journal of Biological Sciences. 23 (2), 257-267 (2016).
- Maschio Rodrigues, M., López Greco, L. S., de Almeida, L. C. F., Bertini, G. Reproductive performance of Macrobrachium acanthurus (Crustacea, Palaemonidae) females subjected to unilateral eyestalk ablation. Acta Zoologica. 103 (3), 326-334 (2022).
- Zhang, C. et al. Changes in bud morphology, growth-related genes and nutritional status during cheliped regeneration in the Chinese mitten crab, Eriocheir sinensis. PLoS One. 13 (12), e0209617 (2018).
- Zhang, C. et al. Hemolymph transcriptome analysis of Chinese mitten crab (Eriocheir sinensis) with intact, left cheliped autotomy and bilateral eyestalk ablation. Fish & Shellfish Immunology. 81, 266-275 (2018).
- Diarte-Plata, G., Sainz-Hernandez, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., Puente-Palazuelos, C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science. 140 (3-4), 172-178 (2012).
- Mirera, D. O., Moksnes, P. O. Comparative performance of wild juvenile mud crab (Scylla serrata) in different culture systems in East Africa: Effect of shelter, crab size and stocking density. Aquaculture International. 23 (1), 155-173 (2015).
- Ut, V. N., Le Vay, L., Nghia, T. T., Hong Hanh, T. T. Development of nursery cultures for the mud crab Scylla paramamosain (Estampador). Aquaculture Research. 38 (14), 1563-1568 (2007).
- Fazhan, H. et al. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Applied Animal Behaviour Science. 247, 105553 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved