דיכאון נקודת הקפאה כדי לקבוע תרכובת לא ידועה
Overview
מקור: המעבדה של לין אוקונל - מכללת בוסטון
כאשר תרכובת מוצקה מומסת בממס, נקודת ההקפאה של הפתרון המתקבל נמוכה מזו של הממס הטהור. תופעה זו ידועה בשם דיכאון נקודת קיפאון, ואת השינוי בטמפרטורה קשור ישירות למשקל המולקולרי של solute. ניסוי זה נועד למצוא את זהותו של תרכובת לא ידועה באמצעות תופעת הדיכאון נקודת הקפאה כדי לקבוע את משקלו המולקולרי. המתחם יתמוסס cyclohexane, ואת נקודת ההקפאה של פתרון זה, כמו גם של cyclohexane טהור, יימדד. ההבדל בין שתי הטמפרטורות הללו מאפשר חישוב המשקל המולקולרי של החומר הלא ידוע.
Principles
תכונות מסוימות של פתרון שונים מאלה של ממס טהור עקב אינטראקציות המתרחשות בין מולקולות ממסים. המאפיינים המציגים שינויים כאלה נקראים המאפיינים הקוליגטיביים וכוללים הורדת לחץ אדים, גובה נקודת רתיחה, דיכאון נקודת הקפאה ושינויים בלחץ האוסמוטי. תכונות אלה תלויות רק במספר החלקיקים המומסים בממס, ולא בזהות החלקיקים. חלקיק, במקרה זה, מוגדר כיון או מולקולה. ניסוי זה מתמקד ברכוש של דיכאון נקודת הקפאה.
כאשר solute מסוים מומס בממס, הביטוי הבא מחזיק נכון:
ΔT = Tf° - Tf = Kfm
המונחים Tf° ו- Tf מתייחסים לטמפרטורות נקודת ההקפאה של הממס הטהור והפתרון, בהתאמה. המונח "m" מציין את המולת של הפתרון, המוגדר כמספר מולים של solute לכל 1,000 גרם של ממס. כמות זו משמשת, ולא טוחנת, כי זה לא תלוי בטמפרטורה. הקבוע, Kf, מכונה קבוע נקודת הקפאה-דיכאון תלוי רק על הממס. שינוי הטמפרטורה תלוי גם במספר החלקיקים ההולכים בתמיסה - ככל שיש יותר חלקיקים, כך השינוי בטמפרטורה גדול יותר. מסיבה זו, המשוואה הקודמת נכתבת לעתים כ:
Tf° - Tf = Kfim
כאשר i = מספר החלקיקים ההולכים המיוצרים ליחידת פורמולה שמתמוססת. בתמיסה המכילה אלקטרוליט, כל יון נחשב לחלקיק.
ניסוי זה משתמש cyclohexane, תרכובת אורגנית כי הוא נוזל בטמפרטורת החדר, כמו ממס. התרכובת הלא ידועה היא מולקולה אורגנית לא יונית; לכן, אני שווה ל- 1. המשקל המולקולרי של תרכובת לא ידועה זו יכול להיקבע על ידי התבוננות בנקודת ההקפאה של פתרון של המתחם cyclohexane והשוואתו לנקודת ההקפאה של cyclohexane טהור.
cyclohexane המתחם יש נקודת התכה (או נקודת הקפאה) של כ 6 °C (6 °F). סדרה של טמפרטורות של cyclohexane טהור מתקבלים כפי שהוא מתקרר מטמפרטורת החדר דרך נקודת ההקפאה שלה באמבט קרח. הטמפרטורות האלה מתוותות אז כפונקציה של זמן. באופן דומה, טמפרטורות של פתרון של המתחם הלא ידוע מומס cyclohexane מתקבלים כפי שהוא מתקרר עד לנקודת ההקפאה, אשר גם שרטטו. העלילות אמורות להיראות דומות לחלקות באיור 1. ניתן לשער את ערכי Tf° ו- Tf, כפי שמוצג. באיור 1b, הטמפרטורה אינה נשארת קבועה לחלוטין כשהפתרון קופא. נקודת ההקפאה של הפתרון היא הנקודה שבה הוא מתחיל להקפיא לראשונה והוא מצוין באופן גרפי על ידי שינוי במדרון של עקומת זמן הטמפרטורה.
הרכיכות, m, של פתרון יכולה לבוא לידי ביטוי במונחים של המסה הטוחנת של solute:
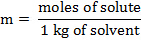
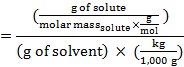
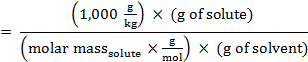
החלפת ביטוי זה במשוואה עבור דיכאון נקודת הקפאה (כאשר i = 1), משיגה:
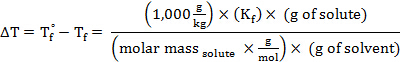
סידור מחדש כדי לפתור עבור מסה טוחנת, משיג:
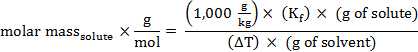
המשקל המולקולרי (באמו) של חומר יש את אותו ערך מספרי כמו המסה הטוחנת שלה.
החומר הלא ידוע הוא אחת התרכובות הבאות:
- ביפניל (C12H10)
- 2-ברומוכלורובנזן (C6H4BrCl)
- נפטלין (C10H8)
- אנתרקין (C14H10)
- 1,4-דיברתברומבנזן (C6H4Br2)
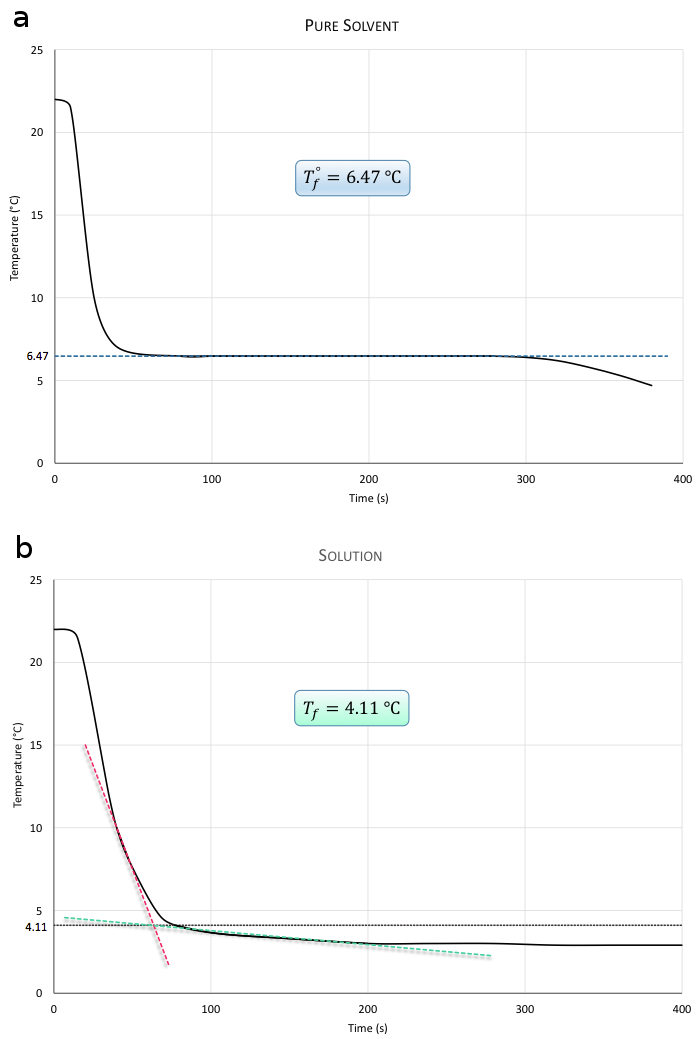
איור 1. איור 1a הוא חלקת טמפרטורה כפונקציה של זמן לקביעת Tf° עבור הממס הטהור. איור 1b הוא חלקת טמפרטורה כפונקציה של זמן לקביעת Tf לפתרון.
Procedure
גשושית טמפרטורה הממשקת למחשב משמשת לרכישת קריאות הטמפרטורה בניסוי זה. בדיקת הטמפרטורה יש אי ודאות של ±0 °C (50 °F).
1. הגדרת הפרמטרים בתוכנה
- הגדר את אורך הניסוי ל-800.
- הגדר את קצב הדגימה לדגימה אחת לשניה.
- הגדר את הגבול העליון עבור טווח הטמפרטורה ל 40 °C (50 °F) ואת הגבול התחתון ל 0 °C (50 °F).
2. מדידת נקודת ההקפאה של Cyclohexane
- יש לחלק 12.0 מ"ל של ציקלוהקסן מהבקבוק המחלק למבחנה נקייה ויבשה.
זהירות: Cyclohexane הוא ממס דליק. - נגב את בדיקת הטמפרטורה עם מגבון ללא מוך כדי לוודא שהוא יבש.
- הכנס את פקק עם בדיקה הטמפרטורה מערבל תיל לתוך המבחנה.
- ודא את קצה הבדיקה הטמפרטורה הוא במרכז הנוזל ולא נוגע בצדדים או בתחתית המבחנה.
- ממלאים 600 מל כשליש מלא במים, ומוסיפים קרח עד שהכוס מלאה בשלושה רבעים.
- התחל את איסוף הנתונים. המחשב רוכש קריאת טמפרטורה בכל שנייה.
- הזז את המבחנה לתוך אמבט מי הקרח והחזק אותה כך שרמת הנוזל במבחנה נמצאת מתחת לרמת המים באמבטיה.
- מיד להתחיל לערבב את הנוזל עם מערבל תיל, ברציפות בקצב קבוע.
- ברגע שההקפאה מתחילה, כל עוד נוזל ומוצק נמצאים שניהם, הטמפרטורה נשארת קבועה עד שהמסה כולה התגבשה. אפשר למחשב להמשיך לרשום את הטמפרטורה עד שהעלילה תתייצב בטמפרטורה קבועה.
שים לב כי ברגע cyclohexane יש קפוא מוצק, הטמפרטורה מתחילה לרדת שוב. - לאחר איסוף מספר מספיק של נקודות נתונים, הפסק את איסוף הנתונים.
- הסר את המבחנה מאמבט מי הקרח ולתת לו להתחמם לטמפרטורת החדר.
- שמור את הנתונים.
- התאם את גבולות ציר ה- yכך שהעיוות תמלא את הדף. כותרת התרשים ולאחר מכן הדפס אותו.
3. הכנת פתרון המתחם הלא ידוע
- שוקלים במדויק 0.14 גרם של החומר המוצק והלא ידוע על פיסת נייר שקילה.
- בדוק כדי להיות בטוח cyclohexane הכלול במבחנה נמס.
- הסר את פקק מן המבחנה בזהירות להוסיף את מוצק לא ידוע cyclohexane, הימנעות אובדן של כל מתחם דבק בצדי המבחנה או פקק.
- החלף את המעצור ושקל מחדש את הנייר כדי להסביר את כל הגבישים שנותרו עליו.
- מערבבים את התמיסה על מנת להמיס לחלוטין את המוצק. חשוב שלא יישארו גבישים.
- הפוך אמבט מים קרח חדש.
4. מדידת נקודת ההקפאה של המתחם הלא ידוע
- הכן את המחשב לאיסוף ערכת נתונים שנייה.
- התחל את איסוף הנתונים.
- העבר את המבחנה המכילה את הפתרון לאמבטיית מי הקרח.
- מיד להתחיל ערבוב הפתרון ברציפות בקצב קבוע.
- לאסוף את הנתונים עבור 300-500 s על מנת לראות בבירור את השינוי במדרון המתרחש כמו הפתרון קופא.
- הפסק את איסוף הנתונים.
- שמור את הנתונים, התאם את גבולות ציר ה- y, תכותרת התרשים והדפס אותו.
- אין להשליך שום ציקלוהקסן או תרכובת לא ידועה במורד הכיור. יוצקים את התערובת הנוזלית לצנצנת "פסולת המעבדה". לשטוף את המבחנה ואת בדיקת הטמפרטורה עם אצטון כדי להסיר את העקבות האחרונים של כל גבישים, לשפוך את השטיפות בצנצנת הפסולת.
Results
ניתן לחשב את המסה של cyclohexane שניתן היה לחלק. הצפיפות של cyclohexane הוא 0.779 g / mL.

ניתן לקבוע את הערכים עבור Tf° ו- Tf מהמגרשים.
ניתן לחשב גם את המסה הטוחנת, ולכן את המשקל המולקולרי של המתחם הלא ידוע. עבור cyclohexane, Kf = 20.2 °C ק"ג / מול של solute.
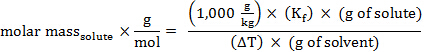
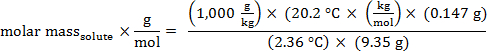
מסת טחנת = 134 גרם/מול
משקל מולקולרי = 134 אמו
המשקלים המולקולריים של התרכובות האפשריות הם:
- 154.21 אמו לביפניל
- 191.46 אמו עבור 2-ברומוכלורובנזנה
- 128.17 אמו לנפתלין
- 178.23 אמו לאנתרקין
- 235.90 אמו עבור 1,4-דיברתולמובנזן
הערך שנקבע באופן ניסיוני עבור המשקל המולקולרי של המתחם הלא ידוע הוא הקרוב ביותר לערך הספרותי עבור נפטלין.
ניתן לחשב את שגיאת האחוז.
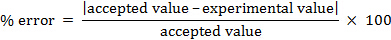
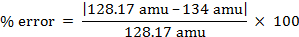
% error = 4.55%
Application and Summary
אולי היישום הבולט ביותר של תופעת הדיכאון נקודת הקפאה מתרחשת במהלך חודשי החורף, כאשר כבישים ומדרכות הופכים קפואים, ומלח משמש לטיפול במשטחים החלקלקים. כאשר המלח מתערבב עם הקרח, נקודת ההקפאה של המים מדוכאת כך שהקרח נמס בטמפרטורה נמוכה יותר. מכיוון שמידת הדיכאון של נקודת ההקפאה תלויה במספר החלקיקים בתמיסה, מלחים המשחררים שלושה יונים ליחידת פורמולה, כגון סידן כלוריד (CaCl2), מנוצלים לעתים קרובות למטרה זו. יצרני גלידה גם לעשות שימוש בדיכאון נקודת הקפאה המתרחשת כאשר מלח וקרח מעורבבים. נקודת ההקפאה של שמנת היא הרבה מתחת 0 °C (50 °F), במיוחד כאשר הוא משולב עם סוכר ומרכיבים אחרים המשמשים להכנת גלידה. מסיבה זו, קרח ומלח סלע משולבים במיכל החיצוני של מכונת גלידה על מנת להשיג טמפרטורה נמוכה מספיק כדי להקפיא את התערובת המצורפת במיכל הפנימי.
כימאים מנצלים את התופעה של דיכאון נקודת הקפאה בניתוח של תרכובות אורגניות מוצקות. הטוהר של מוצר מוצק מסינתזה כימית נקבע לעתים קרובות על ידי מדידת נקודת ההיתוך (תיאורטית, זהה לנקודת ההקפאה) של החומר. אם קיים טומאת תוקף במתחם, נקודת ההיתוך הנצפית נמוכה מהצפוי. זה קורה כי, כמו מוצק מתחיל להינמס, ההטמאה פועלת כמו solute כי הוא מומס בצורה נוזלית של המתחם; לכן, נקודת ההיתוך, או ההקפאה של המתחם מדוכאת.
תעשיית התרופות משתמשת בכמויות גדולות של ממיסים אורגניים לתגובות שמובילות לסינתזה של סוכנים טיפוליים. ממיסים אלה יוצרים כמויות משמעותיות של פסולת נוזלית המסוכנות לסביבה. מדי פעם, ניתן לנצל את תופעת הדיכאון נקודת ההקפאה כדי לחסל את הצורך ממס בסינתזה. כאשר מגיבים מוצקים המעורבים בתגובה נמחצים יחד, נקודות ההיתוך (או ההקפאה) של שתי התרכובות יורדות. אם לשתי התרכובות יש נקודת התכה נמוכה מאוד, הזוג למעשה הופך לנוזלים בטמפרטורת החדר כאשר הם טחונים יחד, מה שמאפשר למולקולות לקיים אינטראקציה זו עם זו כדי שהתגובה תתרחש. תהליכים אלה ללא ממסים הם דוגמה לכימיה "ירוקה", המתייחסת להליכים כימיים המפחיתים או מבטלים את השימוש והיצירה של חומרים מסוכנים.
Skip to...
Videos from this collection:

Now Playing
דיכאון נקודת הקפאה כדי לקבוע תרכובת לא ידועה
General Chemistry
160.8K Views

כלי זכוכית ושימושים נפוצים במעבדה
General Chemistry
658.8K Views

פתרונות וריכוזים
General Chemistry
275.3K Views

קביעת הצפיפות של מוצק ונוזל
General Chemistry
556.8K Views

קביעת הרכב אחוז המסה בפתרון מימי
General Chemistry
383.8K Views

קביעת הנוסחה האמפירית
General Chemistry
183.7K Views

קביעת כללי המסיסות של תרכובות יוניות
General Chemistry
141.6K Views

שימוש במד pH
General Chemistry
346.9K Views

מבוא לתמצית
General Chemistry
425.6K Views

חוק הגז האידיאלי
General Chemistry
79.2K Views

קביעת ספקטרופוטומטריה של קבוע שיווי משקל
General Chemistry
158.8K Views

עקרון לה שאטלייה
General Chemistry
265.8K Views

קביעת חוקי התעריפים וסדר התגובה
General Chemistry
196.3K Views

שימוש בסריקה דיפרנציאלית קלורימטריה למדידת שינויים באנטלפיה
General Chemistry
44.8K Views

מתחמי כימיה של תיאום
General Chemistry
91.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved