קביעת חוקי התעריפים וסדר התגובה
Overview
מקור: המעבדה של ד"ר ניל אברמס — מכללת SUNY למדעי הסביבה ויערנות
לכל התגובות הכימיות יש שיעור מסוים המגדיר את ההתקדמות של מגיבים הולכים למוצרים. שיעור זה יכול להיות מושפע טמפרטורה, ריכוז, ואת המאפיינים הפיזיים של המגיבים. התעריף כולל גם את המתווכים ואת מצבי המעבר שנוצרים אך אינם המגיבים ולא המוצר. חוק התעריפים מגדיר את תפקידו של כל מגיב בתגובה וניתן להשתמש בו כדי לעצב מתמטית את הזמן הנדרש לתגובה כדי להמשיך. הצורה הכללית של משוואת קצב מוצגת להלן:
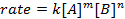
כאשר A ו- B הם ריכוזים של מינים מולקולריים שונים, m ו- n הם סדרי תגובה, ו- k הוא קבוע הקצב. הקצב של כמעט כל תגובה משתנה עם הזמן כאשר המגיבים מתרוקנים, מה שהופך התנגשויות יעילות פחות סביר להתרחש. קבוע הקצב, לעומת זאת, קבוע לכל תגובה בודדת בטמפרטורה נתונה. סדר התגובה ממחיש את מספר המינים המולקולריים המעורבים בתגובה. חשוב מאוד לדעת את חוק התעריפים, כולל קצב קבוע וסדר תגובה, אשר ניתן לקבוע רק באופן ניסיוני. בניסוי זה, נחקור שיטה אחת לקביעת חוק התעריפים ונשתמש בו כדי להבין את התקדמות התגובה הכימית.
Procedure
1. הכנתח' 2ו2 דילולים
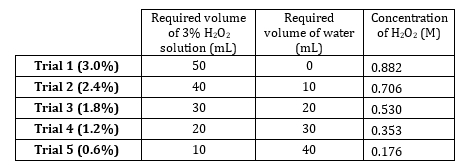
- מלאי 3% מי חמצן יש ריכוז של 0.882 M. להכין 5 דילולים הנעים בין 0.882 M ל 0.176 M (טבלה 1). הכן פתרונות אלה באופן נפחי, אבל להכין אותם תוסף מאז solute הוא מאוד לדלל וכמויות מים הם תוסף.
- מניחים את הפתרונות באמבט מים בטמפרטורה קבועה או משאירים אותם על הספסל כדי להתפרק בטמפרטורת החדר. טווח טמפרטורות של 20°C (293-298 K) טוב לתגובה זו.
.css-f1q1l5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:flex-end;-webkit-box-align:flex-end;-ms-flex-align:flex-end;align-items:flex-end;background-image:linear-gradient(180deg, rgba(255, 255, 255, 0) 0%, rgba(255, 255, 255, 0.8) 40%, rgba(255, 255, 255, 1) 100%);width:100%;height:100%;position:absolute;bottom:0px;left:0px;font-size:var(--chakra-fontSizes-lg);color:#676B82;}
Results
Application and Summary
בעוד שקביעת משתני חוק שיעור יכולה להיות מעורבת מתמטית, השיטות הן למעשה די פשוטות. כל עוד ניתן למדוד את היעלמותו של מגיב או מראה של מוצר, ניתן להשתמש בחלקות שיעור כדי לחשב את קבוע הקצב. הרחבה של שיטה זו משמשת לעתים קרובות כדי לקבוע את אנרגיית ההפעלה של תגובה, Ea, על ידי מדידת הקצב וחישוב ק...
References
- Method adapted from Vetter, T. A., Colombo, D. P. Jr. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide, J. Chem. Ed. 80 (7), 788-798 (2003).
- David R. Lide, ed. CRC Handbook of Chemistry and Physics. Boca Raton, Florida: CRC Press (2005).
Skip to...
Videos from this collection:

Now Playing
קביעת חוקי התעריפים וסדר התגובה
General Chemistry
196.3K Views

כלי זכוכית ושימושים נפוצים במעבדה
General Chemistry
658.8K Views

פתרונות וריכוזים
General Chemistry
275.3K Views

קביעת הצפיפות של מוצק ונוזל
General Chemistry
556.8K Views

קביעת הרכב אחוז המסה בפתרון מימי
General Chemistry
383.8K Views

קביעת הנוסחה האמפירית
General Chemistry
183.7K Views

קביעת כללי המסיסות של תרכובות יוניות
General Chemistry
141.6K Views

שימוש במד pH
General Chemistry
346.9K Views

מבוא לתמצית
General Chemistry
425.6K Views

חוק הגז האידיאלי
General Chemistry
79.2K Views

קביעת ספקטרופוטומטריה של קבוע שיווי משקל
General Chemistry
158.8K Views

עקרון לה שאטלייה
General Chemistry
265.8K Views

דיכאון נקודת הקפאה כדי לקבוע תרכובת לא ידועה
General Chemistry
160.8K Views

שימוש בסריקה דיפרנציאלית קלורימטריה למדידת שינויים באנטלפיה
General Chemistry
44.8K Views

מתחמי כימיה של תיאום
General Chemistry
91.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved
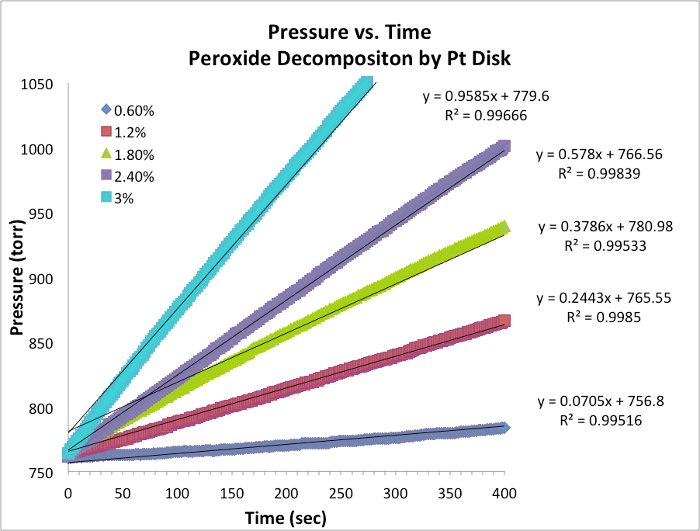
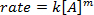 . לקיחת הלוגריתם הטבעי (ln) של המשוואה מייצרת משוואה ליניארית
. לקיחת הלוגריתם הטבעי (ln) של המשוואה מייצרת משוואה ליניארית 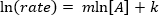 , כאשר m, השיפוע, הוא סדר התגובה.
, כאשר m, השיפוע, הוא סדר התגובה.