סינתזה של קומפלקס קובלט נושא חמצן(II)
Overview
מקור: דיפיקה דאס, תמרה מ. פאוורס, המחלקה לכימיה, אוניברסיטת טקסס A&M
כימיה ביו-אורגנית היא תחום המחקר שחוקר את התפקיד שמתכות ממלאות בביולוגיה. כמחצית מכל החלבונים מכילים מתכות וההערכה היא כי עד שליש מכל החלבונים מסתמכים על אתרים פעילים המכילים מתכת כדי לתפקד. חלבונים הכוללים מתכות, הנקראות מטאלופרוטאין, ממלאים תפקיד חיוני במגוון פונקציות תאים הנחוצות לחיים. מטלופרוטינים סיקרנו והעניקו השראה לכימאים אנאורגניים סינתטיים במשך עשרות שנים, וקבוצות מחקר רבות הקדישו את תוכניותיהן למידול הכימיה של אתרים פעילים המכילים מתכת בחלבונים באמצעות חקר תרכובות תיאום.
ההובלה של O 2 היאתהליך חיוני עבור אורגניזמים חיים. O2 -תחבורהmetalloproteins אחראים כריכה, הובלה, ושחרור חמצן, אשר לאחר מכן יכול לשמש לתהליכי חיים כגון נשימה. קומפלקס תיאום קובלט נושא חמצן, [N,N'-bis (salicylaldehyde)אתילנדימינו]קובלט(II) [Co(salen)]2 נחקר בהרחבה כדי להבין כיצד מתחמי מתכת קושרים באופן הפיך את O2. 1
בניסוי זה, אנו לסנתז [Co(salen)]2 וללמוד התגובה הההפיכה שלה עם O2 בנוכחות דימתילסולפוקסיד (DMSO). ראשית, אנו לכמת את כמות O2 הנצרך עם החשיפה של [Co(salen)]2 ל- DMSO. לאחר מכן נצפה חזותית את שחרורו של O2 מן [Co(salen)]2-O2 להוסיף על ידי חשיפת מוצק CHCl3.
Principles
ישנם שני פולימורפים מוצקים של [Co(salen)]2 (פעיל ולא פעיל), אשר ניתן לבודד מתנאי תגובה שונים. פעיל ולא פעיל [Co(salen)]2 משתנים בצבעם (חום ואדום, בהתאמה), מבנה ותגובה. שני הפולימורפים מורכבים מיחידות דימריק. במקרה של פעיל [Co(salen)]2, המרכזים המשותפים בכל אחת משתי מולקולות Co(salen)2 נמצאים בסמיכות, ויוצרים אינטראקציה חלשה מאוד ואן דר ואלס בין מרכזי המתכת (איור 1). בעוד הצורה הפעילה מציגה אינטראקציה Co-Co חלשה, ההפרדה בין יחידות dimeric מספק מקום O2 להגיב עם מרכזי Co; כתוצאה מכך, הצורה הפעילה של [Co(salen)]2 מגיבה עם O2 במצב מוצק.
בצורה הלא פעילה כביכול של [Co(salen)]2, יש אינטראקציה טיבטיבית בין מרכז Co של מולקולה אחת לבין אטום חמצן מהשנייה(איור 1). שתייחידות Co(salen)קרובות יותר זו לזו בהשוואה לצורה הפעילה, וכתוצאה מכך, הצורה הלא פעילה יציבה באוויר במצב מוצק ומגיבה רק עם O2 בנוכחות ממס מתאם (כגון DMSO), אשר משבש את יחידת dimeric ומייצב את [Co(salen)]2-O2 adduct. לא פעיל [Co(salen)]2 קל יותר להתמודד וללמוד, שכן מוצק יכול להיות מבודד ללא שימוש בטכניקות ללא אוויר. לכן, בניסוי זה אנו לסנתז לא פעיל [Co(salen)]2 וללמוד את התגובה שלה עם O2 בנוכחות DMSO.
ישנן מספר דרכים שבהן O2, מולקולה דיאטומית, יכולה לתאם למרכזי מתכת(איור 2). קשירת סוף התוצאות קשר מתכת חמצן לאחד אטומי חמצן ב O2. בכריכה צדדית, שני אטומי החמצן יוצרים קשרים למרכז המתכת. במקרים מסוימים, יחידת O2 מגשרת על שני מתחמי מתכת שבהם נצפים גם כריכות קצה וצד.
לא פעיל [Co(salen)]2 יוצר קובלט 2:1 ל O2 להוסיף בנוכחות ממס תיאום, DMSO. יחידת O2 מגשרת בין שני מרכזי הקובלט באופן גישור קצה -on (איור 3) ומולקולות DMSO מתואמות להשלים את ספירת התיאום octahedral של כל אחד ממרכזי Co. אם ניקח בחשבון את דיאגרמת MO שלO 2 ו- d-דיאגרמת פיצול מסלולית עבור [Co(salen)]2, אנו יכולים להבין מדוע התוספת 2:1 O2 מועדפת (איור 4). O2 מציג מצב קרקע משולש עם שני אלקטרונים לא משולמים π* MOs. [Co(salen)]2 הוא paramagnetic, עם אלקטרון אחד לא מזווג σ * dz2 MO (בהנחה מטען מרובע (D4h), Co2 +, 7 de-). הכריכה של O2 ל [Co(salen)] 2 היאתגובה redox, שבו שתי מולקולות Co (salen) מחומצנות על ידי 1 e- כל למצב חמצון סופי של +3 בקובלט, ואת מולקולת O2 מופחת על ידי 2 e-,וכתוצאה מכך היווצרות של מי חמצן (O2 2-). התוספת 1:1 אינה מועדפת במקרה זה מכיוון ש- Co(III) הוא d6 ולכן, אינו רוצה לוותר על אלקטרון אחר (לסקירה על תיאוריית MO /d- פיצול מסלולי, ראה את הווידאו על תורת הקבוצה ותורת MO של מתחמי מתכת מעבר).
בסרטון זה, אנו נקבע באופן ניסיוני את יחס Co:O2 על תגובה של לא פעיל [Co(salen)]2 עם O2 בנוכחות DMSO על ידי מדידת הנפח של O2 שאבד במערכת סגורה. אנו יכולים להשתמש בחוק הגז האידיאלי (משוואה 1) כדי לחשב את מספר השומות של O2 הנצרך.
PV = nRT (משוואה 1)
P = לחץ = 1 דולר
V = אמצעי אחסון (L)
R = 0.082 ליטר מול-1 K-1
T = טמפרטורה (K)
n = מולים
לאחר מכן נלמד את הפיך של כריכת O2 על ידי חשיפת מוצק וכתוצאה מכך [Co(salen)]2-O2-(DMSO)2 לכלורופורם (CHCl3). תוספת של CHCl3 (ממס שאינו מתואם שאינו יכול לייצב את [Co(salen)]2-O2 adduct) מוביל לירידה בריכוז של DMSO. העיקרון של לה שאטלייה יכול להסביר כי עם ירידה בריכוז DMSO, שיווי המשקל המוצג באיור 3 יעבור לכיוון המגיבים, וכתוצאה מכך שחרור גז O2.
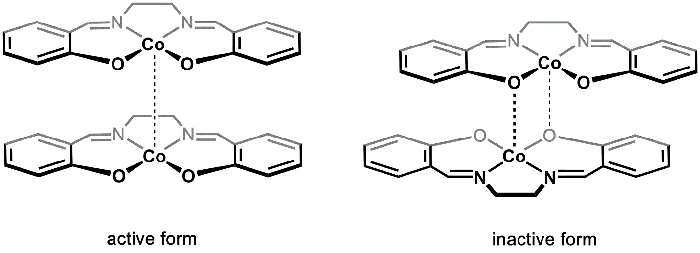
איור 1. צורות פעילות ולא פעילות של [Co(salen)]2.
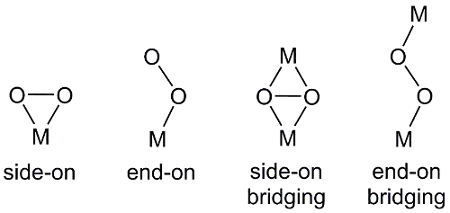
איור 2. מצבי תיאום של O2 למרכז מתכת, M.

איור 3. תגובה הפיכה של O2 עם [Co(salen)]2.
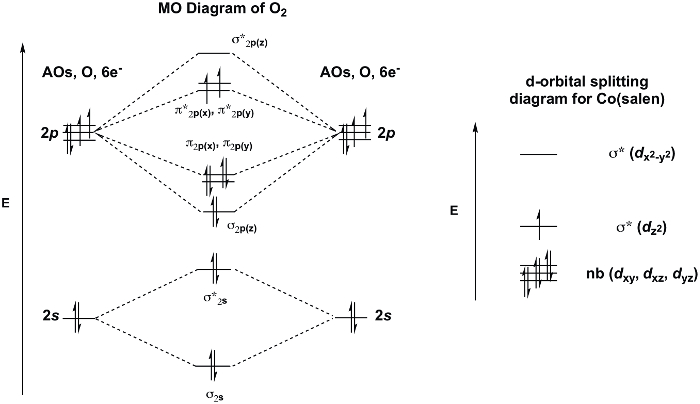
איור 4. דיאגרמת MO שלO 2 ו- d- דיאגרמת פיצול מסלולית של Co(salen) (נגזרת מתיאוריית הקבוצה, בהנחה גיאומטריה מלוכדת מרובעת).
Procedure
1. סינתזה של לא פעיל [Co(salen)]2
- טען בקבוקון עגול 3 צווארים 250 מ"ל עם 120 מ"ל של 95% EtOH ו 2.20 גרם (0.192 מ"ל, 0.018 מול) של סליציללדהיד.
- התאם את הצוואר המרכזי עם מחזק המחובר ל- N2. להתאים את שני הצווארים האחרים עם מחיצת גומי משפך נוסף מצויד במחיצת גומי.
- מערבבים את התגובה באמבט מים ומחממים את הפתרון לרפלוקס (80 מעלות צלזיוס).
- הוסף אתילן דיאמין (0.52 גרם, 0.58 מ"ל, 0.0087 מול) באמצעות מזרק דרך מחיצת הבקבוקון העגולה.
- בבקבוקון עגול 50 מ"ל, להכין פתרון של Co(OAc)2·4H2O (2.17 גרם, 0.0087 מול) ב 15 מ"ל של מים מזוקקים. מחממים את התמיסה באותה אמבטיית מים המכילה את הבקבוקון בעל 3 הצווארים כדי להבטיח שכל הקובלט אצטט יתמוסס.
- מוסיפים את פתרון קובלט אצטט למשפך התוספת.
- Degas פתרון קובלט אצטט על ידי מבעבע N 2 דרך הנוזל במשפך תוספת במשך 10 דקות (ראה "סינתזה של Ti(III) Metallocene באמצעות טכניקת קו Schlenk" וידאו עבורהליך מפורט יותר על טיהור נוזלים). ייתכן שיהיה צורך לסגור את מתאם המרוכז N2 כדי לאפשר ל- N2 לבעבע דרך פתרון הקובלט אצטט.
הערה: לעולם אל תחמם מערכת סגורה! הקפד לפרוק את המערכת במהלך סילוק גיסות. - מוסיפים לאט את תמיסת הקובלט(II) אצטט (~ 1 טיפה/s), תוך ערבוב נמרץ של תערובת האתנול. ללא ערבוב מספיק, ייווצר מזרז גושי שיכול לשבש את מוט הערבוב.
- לאחר כל קובלט אצטט הוסיף, לעורר את התגובה ב reflux במשך 1 שעה.
- כבה את הצלחת החמה והוציא את הבקבוקון העגול בעל 3 הצווארים מאמבט המים.
- מוציאים את המנסרה ומשפך התוספת מהבקבוקון. שקועים בבקבוקון באמבט קרח כדי להקל על משקעים של [Co(salen)]2.
- מסננים את הפתרון תחת ואקום כדי לבודד את המוצק ולשטוף את אדום מוצק וכתוצאה מכך עם אתנול קר.
- לבודד את המוצק. חשב את התשואה של התגובה ולאסוף IR של [Co(salen)]2. ודא כי [Co(salen)]2 יבש לפני השימוש בו בתגובת ספיגת O2.
2. הגדרת מנגנון לספיגת O2 (איור 5)1
הערה: חשוב מאוד שהמערכת לא תדלוף. דליפה במערכת תוביל ליחס Co:O2 נמוך מהצפוי.
- חבר מחט לבלון גז O2 (טוהר גבוה במיוחד) עם צינורות טייגון. בועה בעדינות O2 עד 5 מ"ל של DMSO לפחות 10 דקות.
- בעוד DMSO הוא להיות רווי עם O 2 ,להתאיםאת שני הקצוות של פיפטה זכוכית 10 מ"ל מדורג עם צינורות טייגון (כל 1.5 רגל אורך).
- חבר משפך זכוכית לאחד מחתיכות הצינורות של טייגון.
- מהדקים את פיפטת הזכוכית ואת המשפך לעמדת טבעת כך שהמשפך פונה כלפי מעלה והצינורות יוצרים צורת U (איור 5).
- ממלאים את הפיפטה ומשפך בשמן מינרלי. מוסיפים את השמן דרך המשפך, מוודאים שהשמן גם ממלא את הצינורות המחוברים לפיפטה. ממשיכים להוסיף את השמן עד שהמשפך מתמלא בערך באמצע הדרך במעלה המשפך. אל תתנו לשמן להתקרב יותר מדי לחלק העליון של המשפך, כמו O2 מבעבע דרך המשפך יכול לגרום להתיז אם המשפך מלא מדי.
- לקצה הפתוח של הצינורות, חבר מבחן יד צדדית (מבחנה A).
- הוסף 50 מ"ג (0.077 mmol) של לא פעיל [Co(salen)]2 למבחנה הזרוע הצדדית A מחובר pipette זכוכית.
- הוסף 2 מ"ל של DMSO רווי O2 כדי 3 מ"ל מבחנה (מבחנה B).
- השתמש זוג פינצטה בעדינות להוריד מבחנה B לתוך מבחנה A, נזהר לא לשפוך כל DMSO. חשוב לא לחשוף את [Co(salen)]2 ל- DMSO בשלב זה.
- מבחנה א' עם מחיצת גומי. חוט המחיצה כדי למנוע דליפות.
- הכנס את המחט מחוברת למיכל הדלק O2 לתוך המחיצה לטהר את המערכת עם O2 במשך 10 דקות.
- הסר את מחט O2 ולשמן את החלק העליון של מחיצת גומי כדי למנוע דליפות.
- ייתכן שיהיה צורך לשחרר חלק מהלחץ בתוך ההתקנה כדי להכניס שמן לצינור הזכוכית. כדי לעשות זאת, להכניס מחט חינם לתוך מחיצת גומי במבחנה A. לכסות את הפתח עם אצבע לאט לשחרר את הלחץ בתוך ההתקנה. אל תשכח לכסות את החור החדש עם שומן כדי למנוע דליפות.
- הזז את פיפטת הזכוכית ואת משפך כך רמות השמן בשורה בשתי חתיכות של כלי זכוכית.
- הקלט את עוצמת השמן בתוך פיפטת הזכוכית.

איור 5. מערך ספיגת O2.
3. תגובת ספיגה O2
- הוסף את DMSO למוצק [Co(salen)]2 על ידי בעדינות מפנה את המבחנות, מוודא כי אף אחד מהפתרון נכנס לזרוע הצדדית של מבחנה A.
- לאחר שכל DMSO נוספה, להחזיק את החלק העליון של המבחנה ולערבב בעדינות את הפתרון על ידי ניעור המבחנה הלוך ושוב.
הערה: אין להשתמש בתנועת ניעור מעלה ומטה. דפיקות שתי המבחנות יחד באלימות רבה מדי יכול להוביל לשבירת מבחנה A. - ממשיכים לנער בעדינות את המבחנות ביד עד שרמת השמן בפיפטה מפסיקה לעלות (כ-15-20 דקות).
- לאחר צריכת O2 מפסיק, להזיז את pipette ואת משפך, כך רמות השמן בשורה.
- להקליט את רמת הנפח החדשה של השמן בצינור זכוכית. ההבדל בנפח הוא הנפח של O2 הנצרך במהלך התגובה בלחץ אטמוספרי (1 atm).
- תיעד את טמפרטורת החדר.
4. O2 שחרור מ [Co(salen)]2 - O2 אדוק
- העבר את פתרון DMSO המתקבל מצינור צנטריפוגה 15 מ"ל.
- מלא מבחנה שנייה בכמות שווה ערך של מים.
- הכנס את המבחנות אחד מול השני לתוך צנטריפוגה.
- צנטריפוגה הדגימה לפחות 15 דקות. איכות הכדור המוצק וכתוצאה מכך משתפרת עם הגדלת זמן הצנטריפוגה.
- הסר בעדינות את המבחנה עם [Co(salen)]2-O2 תוספת מדגם, כדי לא להפריע לכדור.
- בזהירות decant פתרון DMSO מעל הכדור.
- מחזיק את צינור הצנטריפוגה בזווית של 45 מעלות עם הכדור פונה כלפי מעלה, לאט להוסיף 1 מ"ל של CHCl3 עם pipette, על ידי מתן פתרון לטפטף במורד הצד של צינור הצנטריפוגה. הזהירות הקיצונית לא להפריע מוצק [Co(salen)]2-O2 להוסיף.
- שים לב לשינויים פיזיים המתרחשים.
Results
אפיון של לא פעיל [Co(salen)]2:
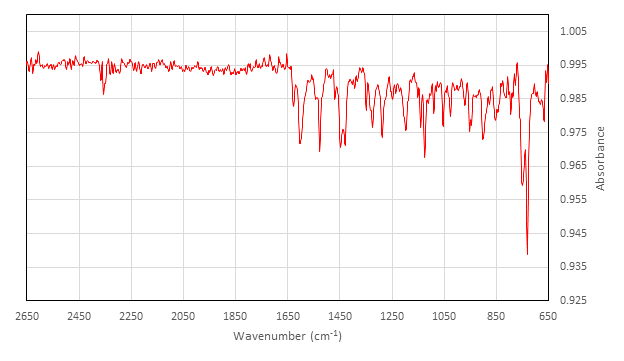
IR (ס"מ-1) שנאסף על קובץ מצורף ATR: 2357 (w), 1626 (w), 1602 (m), 1542 (w), 1528 (m), 1454 (w), 1448 (מ '), 1429 (מ '), 1348 (w), 1327 (w), 1323 (מ '), 12 1248 (מ'), 1248 (w), 1236 (w), 1197 (מ'), 1140 (מ'), 1124 (מ'), 1089 (w), 1053 (מ'), 1026 (w), 970 (w), 952 (w), 947 (w), 902 (מ'), 878 (w), 845 (w), 813 (w), 794 (w), 750 (s), 730 (s).
O2 ספיגה:
59.2 מ"ג (0.090 mmol) של [Co(salen)]2 נצרך 0.002 L של O2. באמצעות לחץ סטנדרטי והטמפרטורה שנרשמה בשלב 3.6, מספר השומות של O2 נצרך היה:

השומות המחושב של Co ב 0.090 mmol של [Co(salen)]2:
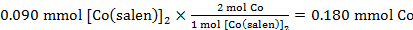
לכן יחס Co:O2 היה:
0.180 mmol Co : 0.082 mmol O2
שווה ערך ליחס של 2:0.91 של Co ל- O2.
תוספת של CHCl3 ל[Co(salen)]2–O2 Adduct:
עם תוספת של CHCl3, פתרון CHCl3 הפך אדום וזרם של בועות שוחרר מן מוצק, המציין שחרור של גז O2 היווצרות של לא פעיל [Co(salen)]2.
Application and Summary
בסרטון זה הסברנו את הדרכים השונות שבהן חמצן דיאטומי יכול לתאם למרכזי מתכת. סינתזנו את קומפלקס הקובלט נושא החמצן [Co(salen)]2 ולמדנו את הכריכה ההפיכה שלו עם O2. באופן ניסיוני הדגמנו כי לא פעיל [Co(salen)]2 קושר באופן הפיך O2 ויוצר 2:1 Co:O2 להוסיף בנוכחות DMSO.
כל בעלי החוליות תלויים בהמוגלובין, מטאלופרוטאין שנמצא בתאי דם אדומים, כדי להעביר חמצן לאיברים נשימתיים כמו גם לרקמות אחרות. בהמוגלובין, החמצן נקשר באופן הפיך לקבוצת heme הכוללת מרכז Fe יחיד המתואם לטבעת הטרוציקלית הנקראת פורפירין(איור 6a). המוגלובין אינו המתכת היחידה נושאת חמצן ואחסון. לדוגמה, רכיכות מחזיקות בחלבון הנקרא המוציאנין, הכולל אתר פעיל של דיקופפר האחראי על הובלת חמצן (איור 6b).
שימוש במינים מולקולריים סינתטיים כדי לדגמן אתרים פעילים במתכת הוא מאתגר בשל ההבדלים המובהקים במבנה האלקטרוני של תרכובת תיאום פשוטה בהשוואה לזה של מתכת מוקפת מבנה-על של חלבונים. כתוצאה מכך, לעתים קרובות קשה לשכפל במדויק את המבנה של האתר הפעיל מטאלופרוטאין. אמנם יש דוגמאות של מתחמי מודל המחקים מבנית אתרים פעילים מתכת, יש פחות דוגמאות של מתחמי מודל דומים מבחינה מבנית המציגים תגובתיות הטבועה metalloenzyme המקומי.
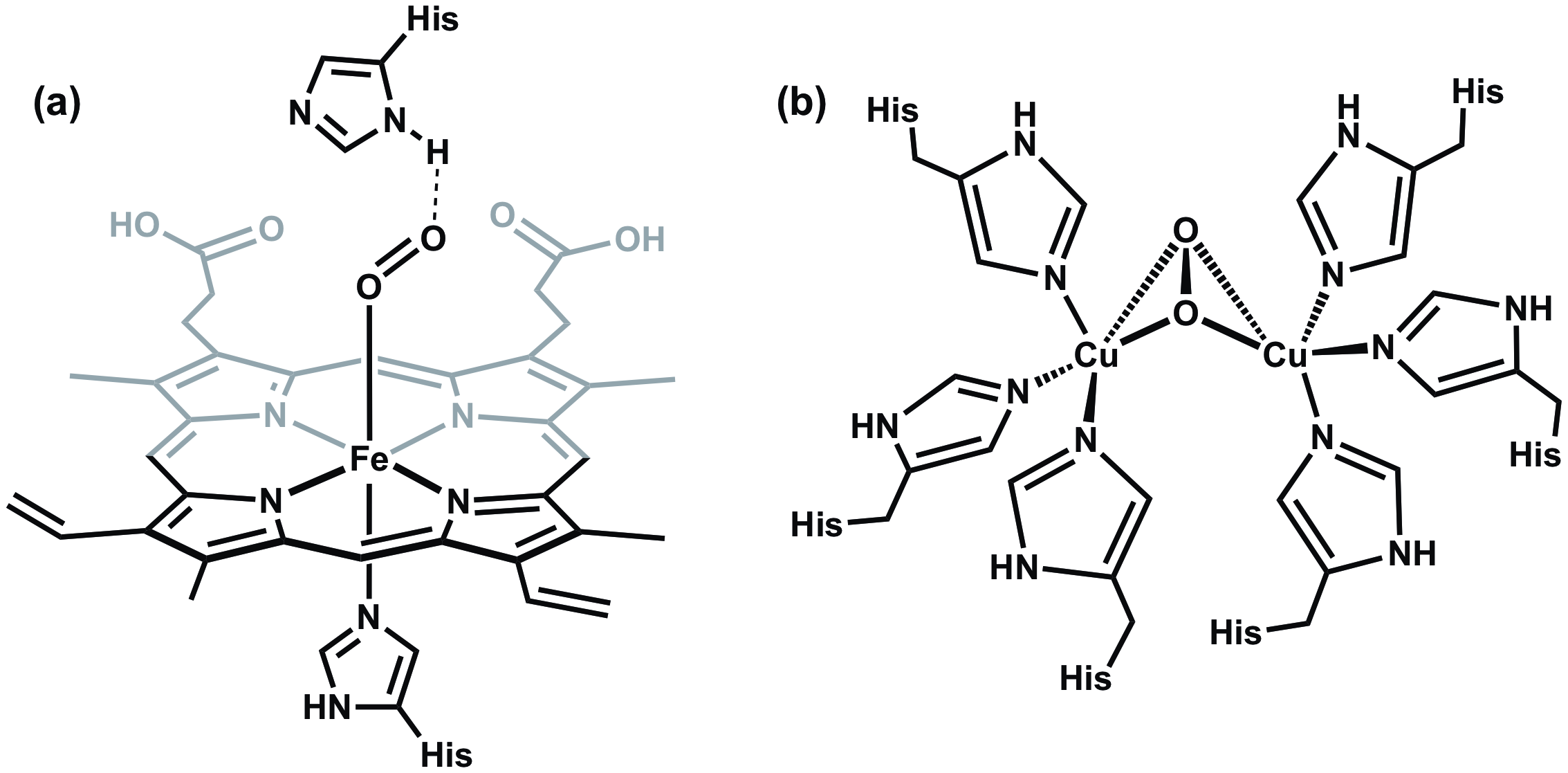
איור 6. (א)מרכז Fe בהמוגלובין נקשר ל- O2 באופן קצה, ואילו (ב)הנחושת המכילה אתר פעיל בהמוסינין נקשרת ל- O2 בכיוון גישור צדדי.
References
- Niederhoffer, E. C., Timmons, J. H., Martell, A. E. Thermodynamics of Oxygen Binding in Natural and Synthetic Dioxygen Complexes. Chem Rev. 84, 137-203 (1984).
- Appleton, T. G. Oxygen uptake by cobalt(II) complex. An undergraduate experiment. J Chem Educ. 54 (7), 443 (1977).
- Ueno, K., Martell, A. E. Infrared Studies on Synthetic Oxygen Carriers. J Phys Chem.60, 1270–1275 (1956).
Tags
Skip to...
Videos from this collection:

Now Playing
סינתזה של קומפלקס קובלט נושא חמצן(II)
Inorganic Chemistry
51.7K Views

סינתזה של מטלוקן Ti(III) בטכניקת קו שלנק
Inorganic Chemistry
31.6K Views

חיישני תא כפפות וטילמאה
Inorganic Chemistry
18.6K Views

טיהור פרוקן על ידי תת-הכרתיות
Inorganic Chemistry
54.7K Views

שיטת אוונס
Inorganic Chemistry
68.6K Views

עקיפה של קריסטל ואבקה
Inorganic Chemistry
104.7K Views

ספקטרוסקופיית תהודה פרמגנטית אלקטרונית (EPR)
Inorganic Chemistry
25.5K Views

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Views

אינטראקציה בסיס חומצה לואיס ב Ph3P-BH3
Inorganic Chemistry
38.9K Views

מבנה פרוקן
Inorganic Chemistry
79.6K Views

יישום תורת הקבוצות לספקטרוסקופיית IR
Inorganic Chemistry
45.5K Views

תורת מסלולית מולקולרית (MO)
Inorganic Chemistry
35.4K Views

גלגלי משוטים מרופדים ממתכת מתכתית
Inorganic Chemistry
15.3K Views

תאים סולאריים רגישים לצבע
Inorganic Chemistry
15.8K Views

ייזום פוטוכימי של תגובות פילמור רדיקליות
Inorganic Chemistry
16.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved