Method Article
Preparation of Agar Bead Embedded Mycobacterium abscessus to Inoculate Immunocompetent Mice Intratracheally
* These authors contributed equally
In This Article
Summary
Mice are generally resistant to infections by Mycobacterium abscessus, which complicates the discovery and development of much-needed antibiotics against pulmonary infections. Here, we describe a method of inoculum preparation and intratracheal infection that was shown to deliver sustained infection in immunocompetent mice.
Abstract
Robust mouse models of chronic infection with Mycobacterium abscessus, an environmental pathogen that preferably infects the lungs, are badly needed. Year-long multidrug therapies deliver unacceptably poor cure rates in patients, around 50%, hence the need for predictive preclinical tools to develop better antibiotics. However, immunocompetent mice are generally resistant to pulmonary infection by M. abscessus. Numerous attempts to establish a sustained infection with M. abscessus in immunocompetent mice have been reported in the literature. Among these, methods relying on agar bead-embedded bacteria inoculated into C57BL/6 mice via the intratracheal route have proven most promising. The major limitation of this approach is the technical challenge associated with the preparation of agar beads of reproducible size and bacterial numbers, followed by intratracheal inoculation. Here, we first provide a detailed description of an optimized protocol that delivers M. abscessus-loaded agar beads of optimal diameter and reproducible bacterial burden. Next, we provide a detailed protocol of intratracheal inoculation, optimized to avoid losses of beads and bacteria due to adherence to surfaces of the inoculation devices and to achieve reproducible pulmonary deposition of agar-embedded M. abscessus. The objective is to ensure ease of implementation and excellent lab-to-lab reproducibility.
Introduction
Treatment options for Mycobacterium abscessus (Mab) pulmonary disease are limited and involve year-long multidrug regimens with oral, parenteral, and occasionally inhaled antibiotics, most of which lack optimal bactericidal activity and are associated with significant toxicity1,2,3,4,5. Shorter, safer, and more effective therapies are urgently needed. Mouse models of mycobacterial infections have been instrumental in the discovery and optimization of effective drugs and drug regimens6,7. However, the preclinical evaluation of antibiotics against Mab infections has proven challenging due to the difficulty of establishing progressive and sustained pulmonary infections in mice8,9.
Infection of immunocompetent mouse strains with Mab largely results in transient colonization followed by rapid clearance of the pathogen10,11. To achieve chronic infection as observed clinically, immobilizing agents such as agarose or agar have been used for promoting the persistence of, and immunopathological responses to, opportunistic pathogens such as Pseudomonas aeruginosa or Haemophilus influenza in immunocompetent mice12,13,14. This model was recently adapted to achieve chronic respiratory infection by Mab ATCC 19977 in immunocompetent C57BL/6N mice15,16,17,18. The method induced persistent infection for up to 45 days after intratracheal inoculation with ~1 × 104 to 1 × 106 colony forming units (CFU), with an incidence of chronic infection among infected mice of 90%-100%19 and the formation of organized granulomas around the disintegrating agar bead18. It appears that embedding Mab in beads protects from rapid clearance by the innate immune system and that beads loaded with bacteria constitute foci for the recruitment of immune cells leading to the formation of granulomas and the establishment of chronic infection18. Agar/agarose beads constitute a relatively inert biomaterial that induces minimal inflammatory responses. In addition, the beads disintegrate slowly, avoiding issues of foreign body allergic reactions or induced fibrosis. Intratracheal infection with or without a microspraying device ensures effective deposition of the inoculum into the lungs8,20,21 and reproduces clinical exposure and infection better than intravenous or intranasal inoculation.
The model, therefore, offers many advantages. However, it also comes with a few challenges of a technical nature, namely (i) achieving reproducible bead size, (ii) obtaining reproducible bead bacterial burden, and (iii) ensuring consistent intratracheal delivery of the inoculum. Here, we provide detailed protocols to reliably achieve these objectives and ensure lab-to-lab reproducibility.
Protocol
All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health with approval from the Institutional Animal Care and Use Committee of the NIAID (NIH), Bethesda, MD. All procedures have been approved by the Institutional Animal Care and Use Committee. All studies involving M. abscessus were performed in a laboratory with biosafety containment level 2.
1. Preparation of agar bead embedded M. abscessus inoculum
- Prepare a pre-culture of M. abscessus ATCC 19977 by inoculating 1.5 mL of optical density (OD600) 1 (using a spectrophotometer) culture into 200 mL of Middlebrook 7H9 medium supplemented with 0.05% (v/v) Tween 80, 0.5% (v/v) glycerol, and 10% (v/v) Middlebrook albumin-dextrose-catalase enrichment (ADC).
- Incubate the cultures in a Roller Bottle at 37 °C for 24 h until reaching the mid-log phase (OD600 = 0.4-0.6).
- Suspend 1.2 g of Tryptic Soy Broth (TSB) in 40 mL of ultrapure water and add 0.6 g of Difco Agar to a final concentration of 1.5%.
- Transfer 60 mL of heavy mineral oil into a 500 mL Erlenmeyer flask and sterilize both the mineral oil and Tryptic Soy Agar (TSA) at 121 °C for 15 min, then equilibrate at 50 °C in an oven.
- Sample 1 mL of the culture and measure the OD600.
- Harvest cells by centrifugation at 3700 g for 15 min at 4 °C. Resuspend cells in 4 mL of 1x Dulbecco's Phosphate-Buffered Saline (DPBS) to achieve an OD600 of approximately 30.
- Mix 3 mL of bacterial suspension with 27 mL of molten TSA pre-equilibrated at 50 °C in a 50 mL centrifuge tube. Mix quickly by vortexing or pipetting.
- Carefully pour the bacteria-agar mixture (30 mL) into 60 mL of mineral oil and place the flask in a secondary container. Quickly set the mixture to spin at medium speed (420 rpm) on a magnetic stirrer set to 50 °C, using a magnetic stirring bar. Ensure that a visible vortex forms in the oil-agar mix. Stir for 6 min at room temperature (RT).
- Cool the mixture by placing ice into the secondary container and continue stirring for 35 min.
- Stop the stirring and let the agar-bead slurry rest for 20 min (replenish the ice as necessary).
- Transfer the slurry mixture into two 50 mL centrifuge tubes and wash 10 times with one volume of DPBS to remove oil and free bacteria (3700 g, 6 min, 4 °C, for the first 5 cycles).
- Combine the agar bead suspensions into one tube at the third wash. During the first 5 washing cycles, use a serological pipet to gently resuspend the beads.
- For the subsequent 5 wash cycles, use the following centrifugation parameters: 2000 g for 5 min, followed by 1000 g for 4, 3, 2, and 2 min, respectively.
- Resuspend the agar beads in DPBS to a final volume of 40 mL, transfer to a 125 mL sterile bottle, and dilute 4x by adding 120 mL of DPBS.
- Pass the suspension through a 200 µm cell strainer.
- Attach the strainer to a sterile 50 mL centrifuge tube. Then, add sample material onto the strainer and filter the bead suspension.
- To facilitate the filtration process, combine a cell strainer, a funnel, a connector ring, and a 50 mL tube into a unit. Assemble the parts in the order of funnel, cell strainer, connector ring, and 50 mL tube, and slowly pull the piston of a 30 mL syringe attached to the connector ring.
- If needed, rinse the strainer from the opposite side with DPBS to wash off any retained larger beads. Concentrate the diluted suspension by spinning at 1000 g for 2 min or allowing the beads to settle by gravity. After harvesting, store the agar beads at 4 °C for up to 1 week. Fresh preparation is recommended for each batch of experiments.
- Pipette 5 µL of the bead suspension onto a glass slide, cover it with a cover slip, and take images at a few random positions using a light or fluorescence microscope with a 10× objective lens. Save the images and measure the bead diameter with ImageJ (desired size range: 200 ± 50 µm).
2. Titration of agar bead embedded M. abscessus inoculum
- Add 1 mL of the bead suspension into an M Tube and aseptically homogenize to release bacteria embedded in beads using a dissociator set to the pre-defined program RNA.02.01.
- Take 100 µL of the homogenate and serially dilute 1:10 to 1 × 10-5 dilution. Spread dilutions on 7H10 agar supplemented with 10% Oleic Acid-Albumin-Dextrose-Catalase (OADC) and 2% glycerol. Enumerate CFU after 3 to 5 days of incubation.
- Adjust the inoculum titer as needed. For example, if each mouse receives 100 µL of bead suspension containing 1 × 105 CFU to 10 mice, a total of 2 mL suspension containing 1 × 106 CFU/mL is prepared plus ~25% to account for losses in syringes.
3. Testing of bead dispensing through the syringe and needle
NOTE: The following steps were included to ensure that beads do not adhere to the surfaces of the syringe or needle. It was found that it is important to use a glass syringe and a 24 G metal feeding needle to prevent the loss of beads due to adherence to plastic surfaces.
- Prepare PBST by adding 1.25 mL of sterile Tween 80 to 500 mL of sterile PBS.
- Add 900 µL of PBST into five M tubes for testing of the agar beads passing through the feeding needle, as well as 900 µL of PBST into 2-mL sterile tubes to prepare -2 to -6 dilutions of each push-through sample.
- Mix the agar bead embedded M. abscessus prepared earlier using a P1000 pipette to ensure all beads are evenly dispersed.
- Attach a 24 G metal feeding needle to the glass syringe and aspirate the agar bead suspension, making sure to avoid the formation of air bubbles.
- Once the syringe is full without air bubbles, place nylon clips or stoppers on the plunger of the syringe, with no space between clips. The stoppers allow for accurate dispensing of 50-100 µL. It is possible to fit up to 6 clips along the plunger.
- Dispense 100 µL of beads ("push-through") into the M tubes in their respective order.
- Once 5 push-throughs have been collected, homogenize the beads using the tissue dissociator adjusted to the RNA.02.01 setting, adequate for RNA preparation from frozen organs and which was found to effectively release M. abscessus from the beads without affecting cell viability.
- Perform serial dilution for the homogenates in the range of -1 to -6. Plate the dilutions on 7H11 agar plates supplemented with OADC. Count CFU after 5 days.
4. Intratracheal inoculation of mice
- Prepare 6- to 8-week-old CD-1 mice.
- Sedate the mice using an isoflurane aerosol exposure system, at a flow rate of 1-5%, as previously described1.
- Restrain the mice using a three-dimensional (3-D) printed tilted stand (custom-designed and produced). The rod in the center of the stand has a suture string that holds the mouse up vertically by its incisors, placing the mouse in a ventral recumbent position with its head raised.
- Attach a metal 24 G animal feeding needle to a glass syringe filled with agar bead embedded M. abscessus. Place nylon clips or stoppers on the plunger of the syringe to infect each mouse with 50 µL of inoculum.
- Once the sedated mouse is restrained on the suture sting, move the tongue to the side of the mouth using a cotton swab applicator.
- Visualize the back of the mouse's mouth to clearly identify the white section in the back of the mouth and target the mouse's trachea.
- Angle the metal gavage needle vertically above the mouse and insert it at the top of the trachea. There might be slight resistance while passing through the epiglottis.
- Slide the gavage needle down into the trachea, remove the nylon clip or stopper, and dispense 50 µL of agar bead embedded M. abscessus.
- Let the mouse rest for 5 min and repeat steps 4.2- 4.8 a second time. The purpose of a two-step inoculation procedure is to ensure reproducible and deep deposition of the full inoculum in the lung.
- Let the infection progress for 24 h, after which the mouse is euthanized to enumerate bacterial deposition in the lungs.
Results
Bead size and burden
To visualize agar beads and their bacterial burden, the mCherry red fluorescent protein was expressed under the constitutive Hsp60 promoter in the Mab type strain ATCC 19977 and embedded this strain in agar beads as described above. Figure 1A shows the range of bead size (scale bar = 200 μm). Figure 1B shows the burden of 6 independent bead preparations, demonstrating the reproducibility of the procedure as described.
Plating of syringe push-through and bacterial implantation following intratracheal inoculation
The intratracheal inoculation was optimized in two steps. CFU/mL were enumerated in the inoculum prior to and following passing through the glass syringe to ensure that no bead or bacterial losses were incurred as the beads passed through the syringe and connected tube. Table 1 shows the initial bacterial titer of two independent bead preparations (trials 1 and 2) and the number of CFU recovered per 100 mL of 'pushed-through' samples from a single syringe load. To avoid bead and bacterial losses, a glass syringe connected to a metal tube is required. Agar beads tend to adhere to plastic surfaces.
CFU enumeration over time in the bead preparation showed that the bacterial burden of the beads remained stable at 4 °C for 1 week, with a ~2-fold loss of bacterial burden per 2 weeks, and unimpaired lung deposition. Next, groups of 5 to 8 mice were infected with three independent bead preparations to test the reproducibility of the intratracheal inoculation procedure. Table 2 shows the number of lung CFUs per mouse, enumerated 24 h post-infection as described.
Lastly, inter-operator reproducibility of infection was assessed by infecting three groups of 10 CD-1 mice, each performed by a different operator. Lung CFUs enumerated 24 h post-infection are shown in Figure 2.

Figure 1: Pilot experiment to assess bead size, visualize and quantify M. abscessus bacterial load. (A) Agar beads containing M. abscessus ATCC 19977 strain, engineered to express the mCherry red fluorescent protein under the constitutive hsp60 promoter. Five µl of bead suspension were spread onto on a glass slide and imaged with a fluorescence microscope equipped with a camera (10x objective). Each red dot is a fluorescent bacterial cell, enclosed within agar beads of diameters ranging from ~70-250 µm. (B) Bacterial burden, in CFU/mL, of 6 individual bead preparations showing the reproducibility of the method. Please click here to view a larger version of this figure.
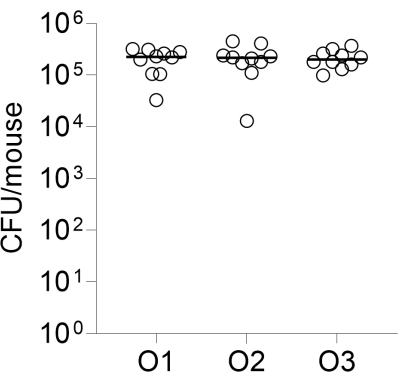
Figure 2: Inter-operator reproducibility of infection. Groups of 10 CD-1 female mice were infected intratracheally by three independent operators as described, using the same bead preparation. Lung CFUs enumerated 24 h post-infection are shown. O1, O2, O3: operators 1, 2, and 3. Please click here to view a larger version of this figure.
| CFU/100 μL | ||
| Bead preparation | Trial 1 | Trial 2 |
| 1.13E+05 | 2.40E+05 | |
| Syringe push-through sample | ||
| 1 | 1.14E+05 | 5.10E+05 |
| 2 | 1.36E+05 | 4.10E+05 |
| 3 | 9.30E+04 | 3.90E+05 |
| 4 | 9.80E+04 | 3.60E+05 |
| 5 | 1.14E+05 | |
| Average | 1.11E+05 | 4.18E+05 |
| SD | 1.69E+04 (15%) | 6.50E+04 (16%) |
Table 1: Colony forming units (CFU) per 100 µL-sample after passing through the glass syringe and metal feeding needle.
| Trial 1 | Trial 2 | Trial 3 | |||
| Mouse ID | Lung CFU/mouse | Mouse ID | Lung CFU/mouse | Mouse ID | Lung CFU/mouse |
| M-1 | 6.50E+03 | M-6 | 2.20E+05 | M-11 | 6.80E+04 |
| M-2 | 1.10E+04 | M-7 | 2.70E+05 | M-12 | 1.70E+05 |
| M-3 | 1.40E+04 | M-8 | 8.10E+04 | M-13 | 2.10E+05 |
| M-4 | 3.20E+03 | M-9 | 1.40E+05 | M-14 | 4.40E+04 |
| M-5 | 4.50E+03 | M-10 | 1.30E+05 | M-15 | 7.50E+04 |
| M-16 | 1.01E+05 | ||||
| M-17 | 7.00E+04 | ||||
| M-18 | 5.80E+03 | ||||
| Average | 7.84E+03 | Average | 1.68E+05 | Average | 9.30E+04 |
| SD | 4.54E+03 (58%) | SD | 7.57E+04 (45%) | SD | 6.67E+04 (72%) |
Table 2: Implantation of agar bead embedded M. abscessus in mouse lungs 24h post-inoculation
Discussion
To ensure the even distribution of bacteria in the agar beads, it is important to uniformly suspend the molten agar-bacteria mixture before adding it to the oil phase. The oil-to-agar-bacteria volume ratio is critical in controlling bead size. A higher oil-to-agar ratio leads to the formation of smaller agar beads. When mixing the agar-bacteria suspension with the oil phase, temperature control is critical. The oil-agar mixture must be warm enough to allow emulsification and droplet formation but cool enough not to harm the bacteria. Continuous stirring while cooling the mixture to room temperature or below allows agar droplets to form and solidify.
Proper calibration of the stirring plate, size of the stir bar, and shape and size of the flask are important determinants of a uniform vortex. A stir bar positioned in the center of the flask creates a symmetric vortex, ensuring uniform mixing. An off-center stir bar can lead to an unstable vortex, resulting in irregularly shaped beads and the introduction of air bubbles into the beads. Real-time monitoring and adjustment of the position of the stir bar is recommended. The volume of the flask needs to be proportional to the volume of the oil-agar mixture. A 500 mL flask is ideal for spinning a 90 mL oil-agar mixture, producing a visible vortex. When using larger flasks, it is important to adjust the volume of the oil-agar mixture, the size of the stirrer, and the vortexing speed to achieve a balance between effective mixing and stable vortex. Maintaining consistent vortexing speed during the process and across bead preparations is critical for batch-to-batch reproducibility.
The agar bead preparation process, specifically the vortexing speed and oil-agar ratio, are adapted from16,22, with minor modifications. Shear forces within the spinning vortex inherently produce beads of different sizes. To minimize this size variability, the bead harvesting step was modified by introducing several washing steps, employing a gradient of decreasing centrifugation speed and time to ensure the removal of small-size beads (and free bacteria). A micro-mesh filter was employed to enrich beads of the desired size, effectively filtering out larger beads and those that may have formed through random coalescence. To avoid bead or bacterial losses as the bead slurry passes through the syringe and connected tube for intratracheal inoculation of the mouse, it is critical to use a glass syringe, 24 G metal feeding needles, and a metal-connected tube.
The major limitation of the method is the technical complexity of the bead preparation and intratracheal inoculation to achieve reproducible bead burden and lung implantation. Attention to detail and the ability to troubleshoot are required. In addition, the model has not been fully validated by testing the efficacy of standard-of-care antibiotics and most frequent antibiotic combinations administered to Mab patients.
Currently, no mouse model of chronic Mab infection has been validated to the extent that the efficacy of single agents and drug combinations in patients can be reasonably predicted by model9. The agar bead-embedded C57BL/6 mouse system has been exploited to test the efficacy of antimicrobial treatments15,19,23. The closest alternative to the agar bead-embedded C57BL/6 mouse system is the C3HeB/FeJ mouse model, which relies on dexamethasone immunosuppression to achieve a durable chronic infection24,25. The advantages of the protocol described here are that (i) fully immunocompetent and most affordable C57BL/6 mice can be used, (ii) a productive and chronic infection can be obtained with the well-characterized type strain ATCC 19977, and (iii) there is no chemically induced immunosuppression requirement, circumventing the need for daily dexamethasone injections, thereby avoiding potential drug-drug interactions and impact on the lung immunopathology.
Given the lack of validated mouse models of chronic Mab infection to evaluate new drug candidates and drug regimens8,9, the technique described here has the potential to move the field forward, improve the predictive value of preclinical efficacy data, help prioritize drug regimens with the highest potential to provide durable cure and overall accelerate drug discovery and development for Mab lung disease. The method can be applied to other lung pathogens and has an established track record for P. aeruginosa or Haemophilus influenza. The major objective of the protocol described here is to provide sufficient details and recommendations to ensure ease of implementation and excellent lab-to-lab reproducibility.
Disclosures
The authors declare no conflict of interest
Acknowledgements
This work was funded by the Cystic Fibrosis Foundation, award DICK24XX0, to TD and VD, and R01-AI132374 from NIH-NIAID to TD and VD.
Materials
| Name | Company | Catalog Number | Comments |
| 125-mL bottle | Corning | 8388 | Sterile |
| 3 x 3 Tube Holding Rack | Fisher | 5972-0030PK | Disinfected prior to use |
| 3D Printed Mouse Stand | Transworld Marketing | custom designed and produced | Disinfected prior to use |
| 48-well dilution Plate | Celltreat | 229192 | Sterile |
| 50-mL centrifuge tubes | Greiner Bio-One | 227261 | Sterile |
| Anesethia Machine | E-Z Systems | EZ-AF9000 SYSTEM | n/a |
| Avanti J-15R | Beckman Coulter | B99517 | Centrifuge |
| Barrier Pipette Tips in Lift-off Lid Rack 1000G | Thermo Scientific ART | 21-236-2A | Sterile |
| Biohazard bag | Fisherbrand | 22-044561 | |
| Connector Ring | pluriSelect | 41-50000-03 | |
| Cotton-Tipped Applicators | Puritan | 22-029-571 | Sterile |
| Disposable Inoculation Loops Yellow Sterile 100 in ziplock bag | Cole-Parmer Essentials | 03-391-562 | Sterile |
| Disposable Poly-Lined Towel Drape | Dynarex | 19-310-671 | Sterile |
| Dulbecco's Phosphate-Buffered Saline | Thomas Scientific | 21-031-CM | Sterile |
| Funnel | pluriSelect | 42-50000 | Sterile |
| GE Ultrospec 10 | Sigma-Aldrich | GE80211630 | Spectrophotometer |
| Gentlemacs Tissue Dissociator | Miltenyi Biotec | 130-096-427 | Not heat activated |
| Hamilton Syringe | Hamilton | 80801 | Disinfected prior to use |
| Heavy Mineral Oil | Sigma-Aldrich | 330760-1L | Sterile |
| Isoflurane solution 250 ml bottle | Covetrus | 29405 | Skin Corrosion/Irritation: Category 2, Serious Eye Damage/Eye Irritation: Category 2A, Specific target organ systemic toxicity (single exposure): Category 3 May cause drowsiness and dizziness, Causes serious eye irritation, Causes skin irritation. Do not inhale, use in well ventilated area. |
| M Tubes (gentleMACS) | Miltenyi Biotec | 130-096-335 | Sterile |
| Magnetic stirrer MR Hei-Connect | Heidolph | 505-40000-13-1 | |
| Magnetic stirring bar | Thomas Scientific | 1181L08 | Sterile |
| Mechanical Pipet 100–1000 µL | Gilson PIPETMAN L | FA10006M | Disinfected prior to use |
| Mechanical Pipet 20–200 µL | Gilson PIPETMAN L | FA10003MG | Disinfected prior to use |
| Metal Animal Feeding Needle 24 G | Braintree Scientific | N-VP 24G-1S | Must be sterilized (autoclaved) |
| Middlebrook 7H10 Agar | Sigma-Aldrich | M0303-500G | Sterilized (autoclaved) |
| Middlebrook 7H11 Agar | Thermo Fisher | R4554002 | Sterilized (autoclaved) |
| Middlebrook 7H9 medium | Beckton Dickinson | 271310 | Sterilized (autoclaved) |
| Mouse Stand Parts | TriMech Solutions | SRV-AMS-FDM | Disinfected prior to use |
| Nonabsorbable Silk Suture | Fisher Scientific | 18020-50 | n/a |
| OADC Liquid Enrichment for use with Middlebrook Media 500 ml | Thermo Scientific Remel | R450605 | Sterile |
| PBS (10x), pH 7.4 Sterile Filtered 500 mL | Gibco | 70-011-044 | Sterile |
| Peroxiguard Wipes | Peroxigard | 29221 | n/a |
| Petri Dishes with Clear Lid Round 100mmx 15mm | Fisherbrand | FB0875712 | Sterile |
| PluriStrainer 200 mm | pluriSelect | 43-50200-03 | Sterile; cell strainer |
| Roller bottles | Corning | 430165 | Sterile |
| Serological pipette | Corning | 4489 | Sterile |
| Spectrophotometer cuvettes 1.5 mL | Fisher Scientific | 14955127 | Sterilized (autoclaved) |
| Syringe stoppers or clips (nylon) | Trimech | SRV-AMS-MJF | Disinfected prior to use |
| Tryptic Soy Broth | Sigma-Aldrich | T8907-500G | Sterile |
| Tween 80 | Fisher | 170793 | Filter sterilized |
| Wide Bore Filtered Pipette Tips Lift-off Lid Rack 200G | Thermo Scientific ART | 21-236-1A | Sterile |
References
- Dartois, V., Dick, T. Drug development challenges in nontuberculous mycobacterial lung disease: Tb to the rescue. J Exp Med. 219 (6), e20220445 (2022).
- Martiniano, S. L., Nick, J. A., Daley, C. L. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med. 43 (4), 697-716 (2022).
- Maurer, F. P., et al. Lack of antimicrobial bactericidal activity in mycobacterium abscessus. Antimicrob Agents Chemother. 58 (7), 3828-3836 (2014).
- Wu, M. L., Aziz, D. B., Dartois, V., Dick, T. Ntm drug discovery: Status, gaps and the way forward. Drug Discov Today. 23 (8), 1502-1519 (2018).
- Egorova, A., Jackson, M., Gavrilyuk, V., Makarov, V. Pipeline of anti-mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities. Med Res Rev. 41 (4), 2350-2387 (2021).
- Nuermberger, E. Using animal models to develop new treatments for tuberculosis. Semin Respir Crit Care Med. 29 (5), 542-551 (2008).
- Nuermberger, E. L. Preclinical efficacy testing of new drug candidates. Microbiol Spectr. 5 (3), (2017).
- Nicola, F., Cirillo, D. M., Lore, N. I. Preclinical murine models to study lung infection with mycobacterium abscessus complex. Tuberculosis (Edinb). 138, 102301 (2023).
- Dartois, V., et al. Preclinical murine models for the testing of antimicrobials against mycobacterium abscessus pulmonary infections: Current practices and recommendations. Tuberculosis (Edinb). 147, 102503 (2024).
- Obregon-Henao, A., et al. Susceptibility of mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother. 59 (11), 6904-6912 (2015).
- Lerat, I., et al. In vivo evaluation of antibiotic activity against mycobacterium abscessus. J Infect Dis. 209 (6), 905-912 (2014).
- Saliu, F., et al. Chronic infection by nontypeable haemophilus influenzae fuels airway inflammation. ERJ Open Res. 7 (1), (2021).
- Rodgers, A. M., et al. Biologically relevant murine models of chronic pseudomonas aeruginosa respiratory infection. Pathogens. 12 (8), 1053 (2023).
- Hoover, J. L., et al. A robust pneumonia model in immunocompetent rodents to evaluate antibacterial efficacy against s. Pneumoniae, h. Influenzae, k. Pneumoniae, p. Aeruginosa or a. Baumannii. J Vis Exp. (119), e55068 (2017).
- Lore, N. I., et al. The aminoglycoside-modifying enzyme eis2 represents a new potential in vivo target for reducing antimicrobial drug resistance in mycobacterium abscessus complex. Eur Respir J. 60 (6), 2201541 (2022).
- Riva, C., et al. A new model of chronic mycobacterium abscessus lung infection in immunocompetent mice. Int J Mol Sci. 21 (18), 6590 (2020).
- Chang, V., Phillips, P. P. J., Imperial, M. Z., Nahid, P., Savic, R. M. A comparison of clinical development pathways to advance tuberculosis regimen development. BMC Infect Dis. 22 (1), 920 (2022).
- Yang, S. J., et al. Pathological granuloma fibrosis induced by agar-embedded mycobacterium abscessus in c57bl/6jnarl mice. Front Immunol. 14, 1277745 (2023).
- Poerio, N., et al. Combined host- and pathogen-directed therapy for the control of mycobacterium abscessus infection. Microbiol Spectr. 10 (1), e0254621 (2022).
- Pearce, C., et al. Inhaled tigecycline is effective against mycobacterium abscessus in vitro and in vivo. J Antimicrob Chemother. 75 (7), 1889-1894 (2020).
- De Groote, M. A., et al. Gm-csf knockout mice for preclinical testing of agents with antimicrobial activity against mycobacterium abscessus. J Antimicrob Chemother. 69 (4), 1057-1064 (2014).
- Facchini, M., De Fino, I., Riva, C., Bragonzi, A. Long term chronic pseudomonas aeruginosa airway infection in mice. J Vis Exp. (85), e51019 (2014).
- Degiacomi, G., et al. The novel drug candidate vomg kills mycobacterium abscessus and other pathogens by inhibiting cell division. Int J Antimicrob Agents. 64 (4), 107278 (2024).
- Rimal, B., et al. T405, a new penem, exhibits in vivo efficacy against m. Abscessus and synergy with beta-lactams imipenem and cefditoren. Antimicrob Agents Chemother. 66 (6), e0053622 (2022).
- Maggioncalda, E. C., Story-Roller, E., Ammerman, N. C., Nuermberger, E. L., Lamichhane, G. Progressive mycobacterium abscessus lung infection in c3heb/fej mice associated with corticosteroid administration. bioRxiv. , (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved