Curve di crescita: generazione di curve di crescita utilizzando le unità formanti colonia e la misurazione della densità ottica
Panoramica
Fonte: Andrew J. Van Alst1, Rhiannon M. LeVeque1, Natalia Martin1e Victor J. DiRita1
1 Dipartimento di Microbiologia e Genetica Molecolare, Michigan State University, East Lansing, Michigan, Stati Uniti d'America
Le curve di crescita forniscono preziose informazioni sulla cinetica di crescita batterica e sulla fisiologia cellulare. Ci permettono di determinare come i batteri rispondono in condizioni di crescita variabili e di definire parametri di crescita ottimali per un determinato batterio. Una curva di crescita archetipica progredisce attraverso quattro fasi di crescita: ritardo, esponenziale, stazionaria e morte (1).

Figura 1: Curva di crescita batterica. I batteri cresciuti in coltura batch progrediscono attraverso quattro fasi di crescita: ritardo, esponenziale, stazionario e morte. La fase di ritardo è il periodo di tempo necessario ai batteri per raggiungere uno stato fisiologico in grado di una rapida crescita e divisione cellulare. La fase esponenziale è lo stadio di crescita e divisione cellulare più veloce durante il quale la replicazione del DNA, la trascrizione dell'RNA e la produzione di proteine avvengono tutte a un ritmo costante e rapido. La fase stazionaria è caratterizzata da un rallentamento e plateau della crescita batterica dovuto alla limitazione dei nutrienti e/o all'accumulo intermedio tossico. La fase di morte è la fase durante la quale si verifica la lisi cellulare a causa di una grave limitazione dei nutrienti.
La fase di ritardo è il periodo di tempo necessario ai batteri per raggiungere uno stato fisiologico in grado di una rapida crescita e divisione cellulare. Questo ritardo si verifica perché ci vuole tempo perché i batteri si adattino al loro nuovo ambiente. Una volta che i componenti cellulari necessari sono generati in fase di ritardo, i batteri entrano nella fase esponenziale di crescita in cui la replicazione del DNA, la trascrizione dell'RNA e la produzione di proteine avvengono tutte a un ritmo costante e rapido (2). Il tasso di rapida crescita e divisione cellulare durante la fase esponenziale è calcolato come il tempo di generazione, o tempo di raddoppio, ed è il tasso più veloce al quale i batteri possono replicarsi nelle condizioni date (1). Il tempo di raddoppio può essere utilizzato per confrontare diverse condizioni di crescita per determinare quale è più favorevole per la crescita batterica. La fase di crescita esponenziale è la condizione di crescita più riproducibile in quanto la fisiologia delle cellule batteriche è coerente in tutta la popolazione (3). La fase stazionaria segue la fase esponenziale in cui la crescita cellulare si stabilizze. La fase stazionaria è causata dall'esaurimento dei nutrienti e / o dall'accumulo di intermedi tossici. Le cellule batteriche continuano a sopravvivere in questa fase, anche se il tasso di replicazione e divisione cellulare è drasticamente ridotto. La fase finale è la morte, dove un grave esaurimento dei nutrienti porta alla lisi delle cellule. Le caratteristiche della curva di crescita che forniscono la maggior parte delle informazioni includono la durata della fase di ritardo, il tempo di raddoppio e la densità massima della cella raggiunta.
La quantificazione dei batteri in coltura batch può essere determinata utilizzando sia le unità formanti colonie che le misurazioni ottiche della densità. L'enumerazione per unità formanti colonie (CFU) fornisce una misurazione diretta della conta delle cellule batteriche. L'unità di misura standard per i CFU è il numero di batteri coltivabili presenti per 1 mL di coltura (CFU/mL) determinato mediante tecniche di diluizione seriale e placcatura diffusa. Per ogni punto temporale, viene eseguita una serie di diluizione 1:10 della coltura batch e 100 μl di ciascuna diluizione vengono distribuiti placcati utilizzando uno spandiconci cella.
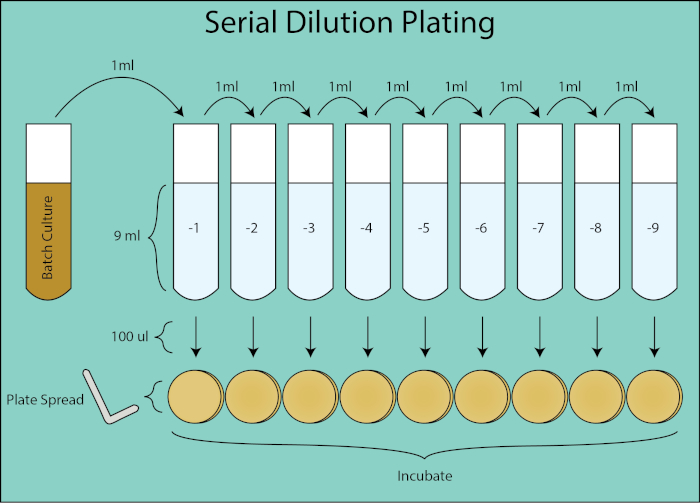
Figura 2. Schema di placcatura a diluizione seriale. Flusso generale per la placcatura di diluizione da coltura batch. La coltura del lotto viene diluita in serie 1:10 trasferendo 1 mL della diluizione precedente nel tubo successivo contenente 9 ml di PBS. Da ogni tubo di diluizione, 100 μl vengono distribuiti placcati utilizzando uno spandipiatti che è una diluizione aggiuntiva di 1:10 in quanto è 1/10 del volume di 1 mL di volume nel calcolo di CFU / mL. Le piastre vengono incubate ed enumerate una volta che le colonie clonali crescono sulle piastre.
Le placche vengono quindi incubate durante la notte e le colonie clonali enumerate. La piastra di diluizione che ha coltivato 30-300 colonie viene utilizzata per calcolare il CFU/ mL per il punto temporale dato (4, 5). La variazione stocastica nel conteggio delle colonie inferiore a 30 è soggetta a un errore maggiore nel calcolo dei CFU/mL e il conteggio delle colonie superiori a 300 può essere sottovalutato a causa dell'affollamento e della sovrapposizione delle colonie. Utilizzando il fattore di diluizione per la piastra data, è possibile calcolare il CFU della coltura batch per ciascun punto temporale.
La densità ottica fornisce un'approssimazione istantanea della conta cellulare batterica misurata utilizzando uno spettrofotometro. La densità ottica è una misura dell'assorbanza delle particelle di luce che passano attraverso 1 cm di coltura e rilevate da un sensore di fotodiodi (6). La densità ottica di una coltura viene misurata in relazione a un mezzo vuoto e aumenta all'aumentare della densità batterica. Per le cellule batteriche, una lunghezza d'onda di 600 nm (OD600) viene in genere utilizzata quando si misura la densità ottica (4). Generando una curva standard relativa alle unità formanti colonie e alla densità ottica, la misurazione della densità ottica può essere utilizzata per approssimare facilmente il conteggio delle cellule batteriche di una coltura batch. Tuttavia, questa relazione inizia a deteriorarsi già a 0,3 OD600 quando le cellule iniziano a cambiare forma e ad accumulare prodotti extracellulari nel mezzo, influenzando la lettura della densità ottica in relazione ai CFU (7). Questo errore diventa più pronunciato durante le fasi stazionarie e di morte.
Qui, L'Escherichia coli viene coltivata in brodo Luria-Bertani (LB) a 37°C nel corso di 30 ore (7). Sono state generate sia curve di crescita CFU/mL che curve di crescita della densità ottica, nonché la curva standard che correla la densità ottica con cFU.
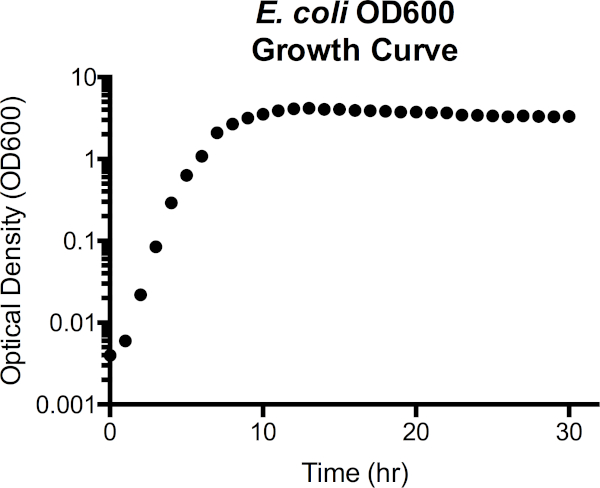
Figura 3. Densità ottica di Escherichia coli alla curva di crescita della lunghezza d'onda di 600 nm (OD600). I valori di densità ottica sono stati prelevati direttamente dallo spettrofotometro dopo l'oscuramento con supporti LB sterili. I valori di OD600 superiori a 1,0 sono stati diluiti 1:10 combinando la coltura di 100 μl con LB fresco di 900 μl, nuovamente misurato, e quindi moltiplicato per 10 per ottenere il valore OD600. Questo passo viene fatto quando la precisione nella misurazione dello spettrofotometro viene ridotta ad alta densità di cellule. Dalla curva, la fase di ritardo si estende a circa 1 ora di crescita, passa alla fase esponenziale da 2h a 7h, quindi inizia a stabilizzarsi, entrando in fase stazionaria. La fase di morte non è una transizione netta, tuttavia, poiché la densità ottica inizia gradualmente a diminuire dopo 15 ore.

Figura 4. Unità formante colonia di Escherichia coli per millilitro (CFU/mL) curva di crescita. I valori di CFU/mL per ogni timepoint sono stati calcolati dalla piastra di diluizione che conteneva 30-300 colonie. Dalla curva, la fase di ritardo si estende fino a circa 2 ore di crescita, passa alla fase esponenziale da 2h a 7h, quindi inizia a stabilizzarsi, entrando in fase stazionaria. La fase di morte non è una transizione netta, tuttavia, poiché CFU / mL inizia gradualmente a diminuire dopo 15 ore da un picco di 2 x 109 a circa 5 x 108 a 30 ore.
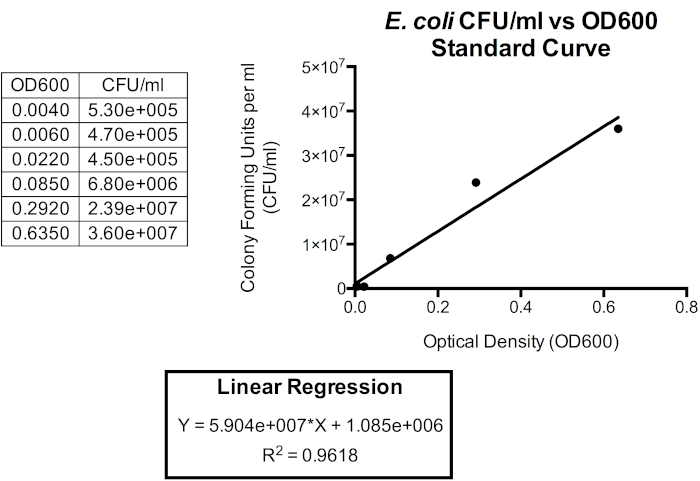
Figura 5. Curva di standardizzazione per CFU/mL rispetto a OD600. Una regressione lineare può essere utilizzata per correlare queste unità in modo che la densità ottica possa essere utilizzata per approssimare la densità cellulare batterica. La densità ottica può essere utilizzata per fornire un'approssimazione istantanea del CFU/mL della coltura batch. Qui, vengono tracciati solo i primi sei punti temporali poiché la relazione tra OD600 e CFU / mL è meno accurata oltre 1,0 OD600 poiché la forma cellulare e i prodotti extracellulari iniziano ad accumularsi quando i batteri entrano in fase stazionaria, che si verifica poco dopo aver raggiunto 1,0 OD600. I cambiamenti nella forma cellulare e nei prodotti extracellulari nei media influenzano la lettura della densità ottica e quindi anche la relazione tra densità ottica e numero di batteri nella coltura è influenzata.
Anche il tempo di raddoppio è stato determinato in 15 minuti e 19 secondi. Da questi dati, la capacità di crescita in LB per E. coli può essere visualizzata ed essere utilizzata per il confronto tra diversi mezzi o batteri.
Procedura
1. Configurazione
- Materiali di laboratorio richiesti: mezzi liquidi, mezzi di agar solidificati, palloni Erlenmeyer, provette da 15 ml, soluzione salina tamponata con fosfato (PBS), diffusore di cellule batteriche, etanolo al 70% e uno spettrofotometro. Tutte le soluzioni e i bicchieri devono essere sterilizzati prima dell'uso.
- Preparare la postazione di lavoro sterilizzando con etanolo al 70%. Lavora vicino a un bruciatore Bunsen per prevenire la contaminazione dei supporti.
Risultati
I grafici delle unità formanti colonie e la densità ottica sono due modi per visualizzare la cinetica di crescita. Determinando la relazione tra CFU/mL e OD600, il grafico a densità ottica fornisce anche una stima di CFU/mL nel tempo. Le condizioni che comportano il più breve tempo di raddoppio sono considerate ottimali per la crescita dei batteri dati.
Applicazione e Riepilogo
Le curve di crescita sono preziose per comprendere la cinetica di crescita e la fisiologia dei batteri. Ci permettono di determinare come i batteri rispondono in condizioni di crescita variabili e di definire i parametri di crescita ottimali per un determinato batterio. I grafici di unità formante colonie e densità ottica contengono entrambi informazioni preziose che descrivono la durata della fase di ritardo, la densità massima cellulare raggiunta e consentono il calcolo del tempo di raddoppio batterico. Le curve di ...
Riferimenti
- R. E. Buchanan. 1918. Life Phases in a Bacterial Culture. J Infect Dis 23:109-125.
- CAMPBELL A. 1957. Synchronization of cell division. Bacteriol Rev 21:263-72.
- Wang P, Robert L, Pelletier J, Dang WL, Taddei F, Wright A, Jun S. 2010. Robust growth of Escherichia coli. Curr Biol 20:1099-103.
- Goldman E, Green LH. 2015. Practical Handbook of Microbiology, Third Edition. CRC Press.
- Ben-David A, Davidson CE. 2014. Estimation method for serial dilution experiments. J Microbiol Methods 107:214-221.
- Koch AL. 1968. Theory of the angular dependence of light scattered by bacteria and similar-sized biological objects. J Theor Biol 18:133-156.
- Sezonov G, Joseleau-Petit D, D'Ari R. 2007. Escherichia coli physiology in Luria-Bertani broth. J Bacteriol 189:8746-9.
Vai a...
Video da questa raccolta:

Now Playing
Curve di crescita: generazione di curve di crescita utilizzando le unità formanti colonia e la misurazione della densità ottica
Microbiology
296.5K Visualizzazioni

Creazione di una colonna di Winogradsky: un metodo per arricchire le specie microbiche presenti in un campione di sedimento
Microbiology
129.5K Visualizzazioni

Diluizioni seriali e piastratura: la conta microbica
Microbiology
316.3K Visualizzazioni

Culture di arricchimento: coltura di microbi aerobici e anaerobici su terreni selettivi e differenziali
Microbiology
132.1K Visualizzazioni

Colture pure e piastratura per striscio: isolamento di singole colonie batteriche da un campione misto
Microbiology
166.2K Visualizzazioni

Sequenziamento dell'rRNA 16S: una tecnica basata sulla PCR per identificare le specie batteriche
Microbiology
189.1K Visualizzazioni

Test di suscettibilità agli antibiotici: test dell'epsilometro per determinare i valori MIC di due antibiotici e valutare la sinergia antibiotica
Microbiology
93.8K Visualizzazioni

Microscopia e colorazioni: la colorazione di Gram, delle endospore e del capside
Microbiology
363.5K Visualizzazioni

Saggio delle placche: un metodo per determinare il titolo virale in unità formanti placca (UFP)
Microbiology
186.3K Visualizzazioni

Trasformazione di cellule di E. coli tramite l'utilizzo di una procedura basata sul metodo del cloruro di calcio
Microbiology
86.9K Visualizzazioni

Coniugazione: un metodo per trasferire la resistenza all'ampicillina dal donatore al ricevente E. coli
Microbiology
38.3K Visualizzazioni

La trasduzione batterica tramite fagi: un metodo per trasferire la resistenza all'ampicillina da una cellula donatore di E. coli ad una ricevente
Microbiology
29.1K Visualizzazioni
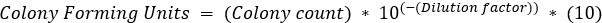
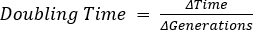
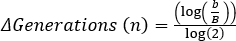 , dove b = numero di batteri a t2, B = numero di batteri a t1e n = numero di generazioni. Derivato da:
, dove b = numero di batteri a t2, B = numero di batteri a t1e n = numero di generazioni. Derivato da: