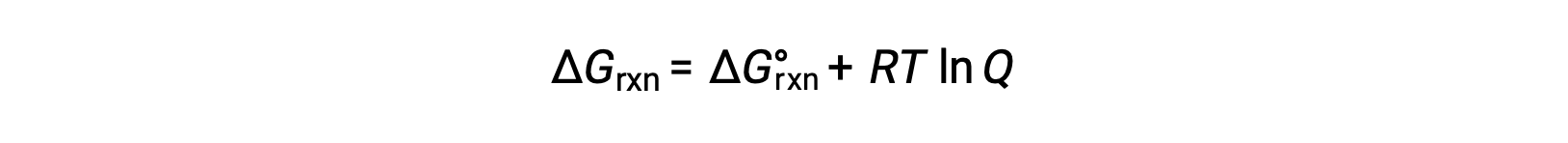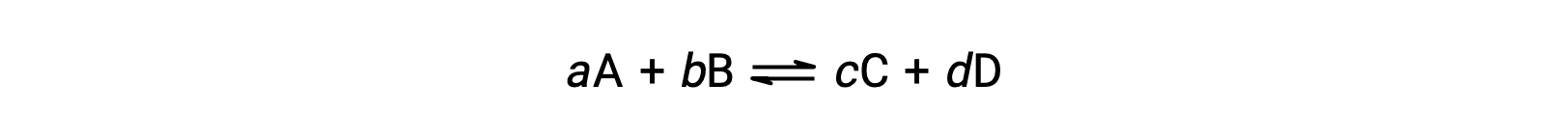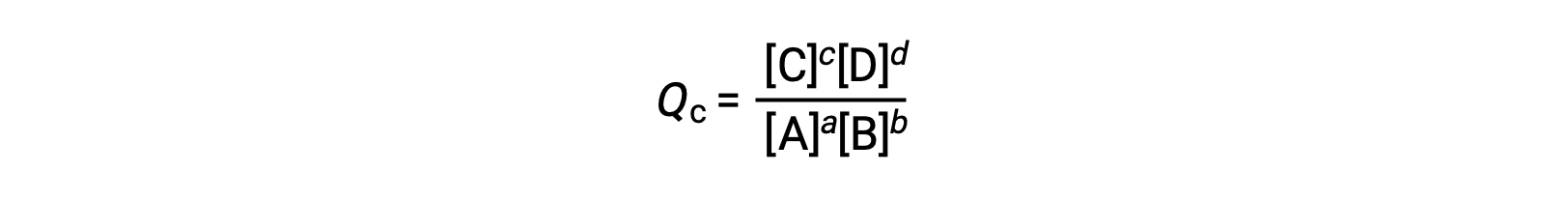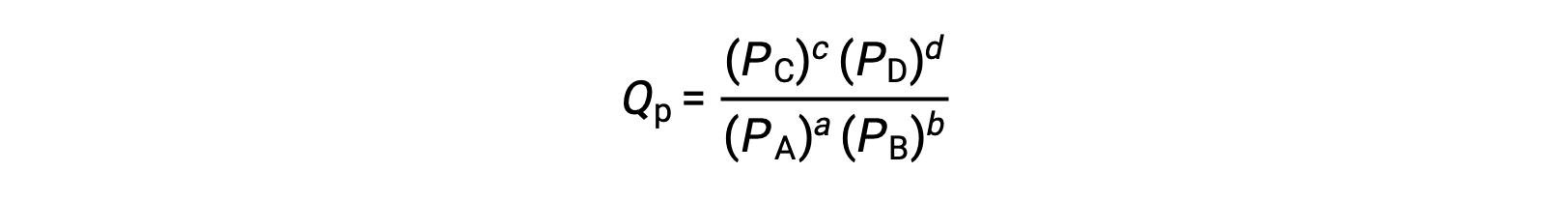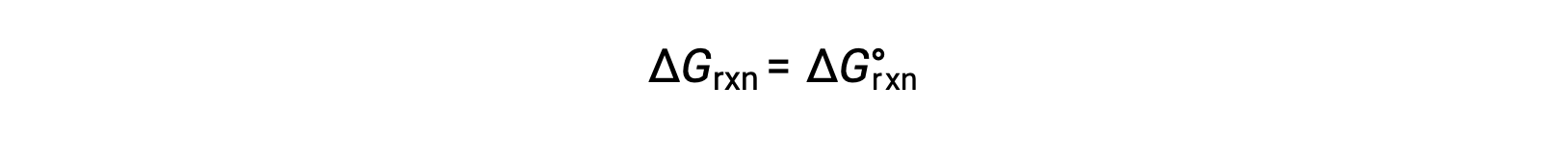The standard free energy change for a reaction can only be determined if it occurs under standard state conditions—when both the reactants and products are in their standard states. However, most chemical reactions do not occur under these conditions.
Under any conditions—standard or nonstandard—the relative amount of products and reactants present in a reaction is described by the reaction quotient, Q.
For reactions occurring in solution, Q is calculated from the ratio of the product and reactant concentrations, with each reagent concentration raised to the power of its stoichiometric coefficient. For gaseous reactions, the partial pressures of the gases can be used in place of concentrations.
The free energy change of a reaction is equal to the sum of the standard-state free energy change for the reaction, delta G naught, and RT times the natural log of Q. Here, R is the universal gas constant in joules per mole-kelvin, and T is the temperature of the reaction in kelvin.
At constant temperature, the standard-state free energy has a fixed value, but Q varies because it depends on the composition of the reaction mixture.
Consider the synthesis of ammonia gas from nitrogen and hydrogen at 298 K. Under standard conditions, which for a gas is the pure gas at 1 atm, the partial pressures of all the components are equal to 1 atm, and the magnitude of the reaction quotient equals 1.
Thus, the free energy change of the reaction is equal to the standard free energy change of the reaction, −32.8 kJ/mol, and the forward reaction is spontaneous.
Under nonstandard conditions, the components of the reaction mixture may initially have partial pressures of 1.2 atmospheres of nitrogen, 3.6 atmospheres of hydrogen, and 0.60 atmospheres of ammonia.
As before, the reaction quotient can be determined from the values for the partial pressures. Substituting for Q into the equation, the free energy for the reaction is −45.3 kJ/mol, indicating a spontaneous reaction in the forward direction.
As the forward reaction proceeds, more ammonia is produced, and the reaction composition changes.
When the reactants and products are in equilibrium, the free energy change for the reaction is zero, and the value of RT times the natural log of Q is equal and opposite in sign to the standard free energy change.
Now, if the reaction mixture contains 0.02 atmospheres of nitrogen, 0.06 atmospheres of hydrogen, and 4.8 atmospheres of ammonia, Q is much larger, and the change in free energy is 5.6 kJ/mol.
A positive free energy change indicates that the reverse reaction is energetically favorable. Thus, under these conditions, ammonia decomposes to produce nitrogen and hydrogen.