このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
Advanced Glycationの最終製品は、カプサイシン誘導性カルシウム流入に対してヒトの感覚様ニューロン細胞を感作します
* これらの著者は同等に貢献しました
要約
コラーゲン由来の終末糖化産物(AGEs)の増加は、一貫して痛みを伴う疾患に関連しています。ここでは、糖化がカプサイシンの興奮に対して感覚ニューロンを感作するかどうかを調べました。
要約
コラーゲン由来の終末糖化産物(AGEs)の増加は、変形性関節症、糖尿病性ニューロパチー、神経変性疾患などの痛みを伴う疾患と一貫して関連しています。SH-SY5Y細胞株から分化したヒト感覚様ニューロンは、AGEsに曝露されると、サブスタンスPを放出し、一過性受容体電位バニロイド1(TRPV1)の発現をアップレギュレーションすることにより、侵害受容促進機能を獲得します。ここでは、この受容体が機能的に活性であるかどうか、また糖化の過程で感覚神経細胞がカプサイシンの興奮に対して感作されるかどうかを調べました。感覚様ニューロン細胞は、SH-SY5Y細胞とオールトランスレチノイン酸および脳由来神経栄養因子との分化から得られた。糖化コラーゲン細胞外マトリックス(ECM-GC)とのインキュベーションは、侵害促進刺激をシミュレートしました。コントロール細胞は、非糖化細胞外コラーゲンマトリックス(ECM-NC)とインキュベートしました。Fluo-8 Calcium Flux Assay Kitは、カプサイシンによって刺激されたカルシウム流入を評価するために使用されました。その結果、糖化は正常なコラーゲンで処理した細胞と比較してカルシウム流入を増加させることが示され、感覚様ニューロンが機能的なTRPV1チャネルを発現し、糖化がカプサイシンの興奮を増加させることが示唆されました。これらのデータは、AGEsが知覚過敏な感覚様ニューロン細胞を示しており、侵害受容促進シグナル伝達を誘発します。まとめると、私たちの結果は、カプサイシンに応答する機能モデルを確立し、痛みを伴う状態を管理するための候補者のスクリーニングに役立つ可能性があることを示唆しています。
概要
糖化は、コラーゲンなどのタンパク質が還元糖分子に結合し、その結果、進行性糖化最終産物(AGEs)が生じる非酵素的、不可逆的、自発的なプロセスです。AGEsは、細胞外シグナル制御プロテインキナーゼ(ERK)1/2、p38マイトジェン活性化プロテインキナーゼ(MAPK)、c-jun n末端キナーゼ(JNK)、rho-GTPアーゼ、ホスホイノシトール-3-キナーゼ(PI3K)、ヤヌスキナーゼ/シグナルトランスデューサーおよび転写活性化因子(JAK/STAT)、プロテインキナーゼC(PKC)などの細胞内経路の活性化を誘発し、炎症誘発性分子の放出と酸化ストレスを増加させる細胞膜受容体を活性化する可能性があります1.また、糖化コラーゲンは細胞外マトリックスの構造と特性を損ない、コラーゲン由来のAGEsの増加は、変形性関節症、糖尿病性ニューロパチー、神経変性疾患などの痛みを伴う疾患と一貫して関連しています2,3。
私たちのグループは以前に、SH-SY5Y細胞株が、ナトリウムチャネル(Nav 1.7、Nav 1.8、およびNav 1.9)や一過性受容体電位バニロイド1型(TRPV1)など、末梢感覚ニューロンに通常見られるマーカーである侵害受容に関与するチャネルを発現するため、感覚様ニューロン細胞に分化できることを示しました4.TRPV1は、カルシウムイオンを透過し、カプサイシン刺激に敏感な非選択的カチオンチャネルです。重要なことは、感覚様ニューロン細胞が糖化コラーゲンマトリックス(ECM-GC)にさらされると、ニューロンの活性化に関与する転写因子であるc-Fos発現と、神経炎症や痛みに広く関与する神経ペプチドであるサブスタンスP放出を増加させることにより、侵害受容促進機能を獲得することです。これらの細胞は、プロトタイプのアヘン剤であるモルヒネなどの鎮痛薬に反応し、ECM-GC誘導性物質Pの放出を減少させます。まとめると、これらのデータは、このモデルが予防分子と抗侵害受容分子に応答することを示しています4,5。
細胞内Ca2+ 濃度変化のモニタリングは、多数の細胞プロセスを研究するために不可欠です。ニューロンでは、薬物のニューロンの損傷や神経保護特性を予測するための有用なツールとなり得ます。唐辛子の辛味成分であるカプサイシンは、TRPV1受容体5 の最も研究されているアゴニストであり、痛みのメカニズムを研究し、潜在的な新しい鎮痛薬をスクリーニングするための貴重なツールです。これまでの研究では、高グルコースでインキュベートされたげっ歯類の後根神経節から採取された一次感覚ニューロンが、カプサイシン誘発性カルシウム流入の有意な増加を示すことが示された6。しかし、TRPV1チャネルが私たちの細胞モデルで機能的に活性であったかどうか、および糖化コラーゲンが感覚様ニューロン細胞をカプサイシン興奮に感作するかどうか、侵害受容シグナル伝達経路を活性化する可能性があるかどうかは不明のままです。そこで、感覚様細胞のカルシウムをリアルタイムでモニタリングするためのシンプルなツールを活用しながら、信頼性の高い解析を行いながら、費用対効果の高いプロトコールを開発することを目指しました。ここでは、研究者が感覚様ニューロン細胞でSH-SY5Y細胞を分化するための手順と、侵害受容促進刺激に対してSH-SY5Y細胞を感作する方法を支援するための包括的なプロトコルを提供します。この方法は、新しい鎮痛剤または神経保護化合物の発見に貢献することができます。
プロトコル
1. SH-SY5Yの培養と感覚様ニューロン細胞への分化
注1:このセクションにあるすべてのステップは、層流フードの下で行う必要があり、すべての溶液と供給品は無菌である必要があります。
- まず、培地5mLを加えて培養フラスコ(25cm2)を調製します。
注:この細胞型の解凍、増殖、および維持のために、培養培地の混合物を使用してください:10%熱不活化ウシ胎児血清と1%ペニシリン-ストレプトマイシンを添加したダルベッコ改変イーグルスおよびハム培地F12(DMEM / F12)。 - 次に、SH-SY5Y細胞が入ったクライオチューブを液体窒素から取り出し、氷上に保ちます。細胞を37°Cの水浴で2分間解凍します。
- クライオバイアルに1 mLの完全培地を加え、細胞を15 mLのチューブに移し、3 mLの完全培地(DMEM/F12に10%熱不活化ウシ胎児血清を添加)を加え、ピペットを使用してチューブに引き上げて分注することを繰り返して混合します。
- 細胞を15mLチューブにピペットで移し、290gで遠心分離します。上清を捨てて、凍結細胞中の氷晶形成を防ぐために使用される凍結保護剤であるジメチルスルホキシド(DMSO)を除去します。
- 細胞ペレットに培地1mLを加え、ピペットでよく混ぜ合わせ、25cm2の培養フラスコに入れて細胞を増殖させます。細胞をインキュベーターに入れ、5% CO2 の加湿雰囲気、37°Cで下します。
注:細胞は、約80%のコンフルエンスに達すると使用できます。ニューロンの分化には、細胞継代20までのSH-SY5Y細胞を使用することをお勧めします。 - ラットテールI型コラーゲンを滅菌PBSで希釈して細胞外コラーゲンマトリックス(100 μg / mL)を調製し、この溶液1 mLをペトリ皿(35/10 mm)に注いでコーティングします。
- これをインキュベーター内で5%CO2 の加湿雰囲気で約1時間インキュベートします。
- この期間の後、滅菌PBSでプレートを2回洗浄し、ノイバウアーチャンバーを使用して細胞をカウントします。
- 細胞をカウントするには、まず細胞を含む25 cm2 の培養フラスコを5 mLの滅菌PBSで洗浄し、すべての培地を取り除きます。次に、滅菌PBSを取り出し、3mLの0.05%トリプシンを細胞に加え、37°Cおよび5%CO2 のインキュベーターに約3〜5分間置いて、細胞の剥離を完了させます。
- その後、インキュベーターから細胞を取り出し、培養フラスコに6 mLの培地を加え、この溶液を15 mLのチューブに移します。290 g で5分間遠心分離します。
- 上清を取り除き、細胞を1 mLの完全培地にしっかりと懸濁します。
- 最後に、ノイバウアーカウンティングチャンバーを使用して細胞をカウントします。20 μLの細胞と20 μLのトリパンブルーを混合し、この混合物10 μLをノイバウアーチャンバーに加えます。青色に染色されていない生細胞を数えます。
- 5 × 10個の 4 細胞/mL 細胞を、前述の同じ培地上のシャーレ (35/10 mm) にプレートします。プレートあたり1mLの総容量を使用してください。
- 次に、24時間待ってニューロン分化プロトコルを開始します。そのためには、培地を取り出し、2%熱不活化ウシ胎児血清、1%ペニシリン-ストレプトマイシン、および10μMオールトランスレチノイン酸(RA 10 μM)を添加した1 mLのDMEM/F12と交換します。
- 48時間待ってから、3日間毎日培地を交換します。細胞をインキュベーターに入れ、5% CO2 の加湿雰囲気、37°Cで下します。
- 5日目に、培地を細胞から取り出し、ヒト脳由来神経栄養因子(BDNF 50 ng/mL)を添加した無血清培地1 mLを各プレートに加えます。
注:さまざまな企業のBDNF(資料の表を参照)がテストされました。彼らは皆、同じように働きました。 - 分化7日目に、細胞を滅菌PBSで一度洗浄し、感覚様ニューロン細胞を実験に用います。これらの細胞をすぐに実験に使用することが不可能な場合は、無血清の培地を追加して、使用するまで細胞を生き生きとさせてください。
注:ニューロンの分化の終わりには、細胞毒性を避けるために、残りのBDNFを除去するために滅菌PBSで細胞を洗浄する必要があります。
2. 糖化コラーゲンと糖化プロセス
注:このセクションのすべての手順は、層流フードの下で行う必要があり、すべての溶液と供給品は滅菌されている必要があります。
- コラーゲン糖化を調製するには、ラットテールフィブリル状態(3.89 mg/mL)のI型コラーゲンを200 mM D-リボース、160 mM D-グルコース、および200 mM D-スレオスと4°Cで7日間インキュベートします。
注:糖化コラーゲンを調製するには、分子量に基づいて糖を秤量し、ラット尾線維状態(3.89 mg / mL)のI型コラーゲンと混合します。 - 血清および抗生物質を補給せずにDMEM/F12培地で調製した糖化コラーゲン細胞外マトリックス(ECM-GC、100 μg/mL)または正常コラーゲン細胞外マトリックス(ECM-NC、100 μg/mL)を用いて、感覚様ニューロン細胞培養を24時間インキュベートします。
3. カルシウム流入アッセイ
- カルシウム流入については、カルシウムフラックスアッセイキットを使用し、以下に示すように、製造元の指示に従って溶液を調製します。
- 最初に、1x assay buffer (HBSS buffer と 10x Pluronic F127 Plus の混合物) 998 μL に 2 μL の Fluo-8 ストック溶液を加えて、1 mL の Fluo-8 色素ローディングを調製します。
注:この作業溶液は、室温(RT)で少なくとも2時間安定です。 - その後、この溶液250 μLを、以前に分化させてECM-NCまたはECM-GCで処理した感覚様ニューロン細胞の各プレーティングディッシュに、37°Cで5%CO2 の加湿雰囲気で30分間インキュベートします。 次に、インキュベーターから皿を取り出し、RT(暗闇)で30分間保管します。
注:この溶液は、2時間のインキュベーションを外挿すると細胞毒性になります。インキュベーション期間中にカプサイシン溶液を調製します。
4.カプサイシン誘導
注:TRPV1アゴニストであるカプサイシンを使用して、細胞内のカルシウム流入を誘導しました。
- カプサイシンを1%エタノールと超純水で秤量し、希釈します。次に、2μMの濃度の溶液を調製し、これも超純水で希釈しました。
注:カプサイシン溶液は使用日に作成する必要があります。カプサイシンを最初にエタノールで希釈し、次に水で希釈します。さもなければ、それは沈殿します。 - 250 μLの2 μMカプサイシン溶液を各35/10 mmシャーレに添加し、最終濃度が1 μMになるようにします。
注:カプサイシンのストック溶液濃度は、より良好な分散を確保し、孤立した蛍光ピークを避けるために 2 μM でした。
5. カルシウム流入イメージングと共焦点顕微鏡分析
注:イメージングは、20x/0.75NA対物レンズと488nm励起レーザー(0.5%強度)を備えた共焦点顕微鏡で行いました。発光は520nmで検出されました。細胞は、xy軸(512×512ピクセル)で時間(t)に600Hzの速度で、取得間隔は433ms、合計取得時間は5分でスキャンしました。イメージングは、顕微鏡ソフトウェアを使用して細胞の生理学的状態を維持するために37°Cで行いました。
- 250 μL の 2 μM カプサイシン溶液 (プレート内の最終濃度は 1 μM になります) でシリンジを準備し、頭皮静脈セット - Butterfly type 23 GA sterile に接続します。ラボスタンドとクランプを使用してシリンジを固定します。
- ペトリ皿を顕微鏡ステージに置き、細心の注意を払って頭皮の針をペトリ皿に入れ、底との接触を避け、針の先端が液体に沈まないようにします。
注意: 粘着テープを使用して頭皮の位置を固定できます。 - 適切な数のセル(20以上)を持つフィールドを見つけ、明視野光を使用してフォーカスを調整します。
- 488 nmレーザーで ライブ モードを開始し、フォーカスと照明のパラメーターを調整します。
- 取り込み記録を開始し、ベースライン期間(t = 0〜t = 60秒)後、250 μLの2 μMカプサイシンを塗布し、シリンジプランジャーを静かに押して、細胞へのカルシウム流入を刺激します。
注:最終的な記録取得時間は、プレートあたり約300秒でした。
6. 後処理/データ分析
- 顕微鏡ソフトウェアを使用して、 定量 モードで細胞解析を行います。まず、カプサイシン刺激の前後の蛍光スパイク細胞を解析します。
- 各応答性細胞、すなわちカプサイシン後に蛍光スパイクを示す細胞に関心領域(ROI)を作成します。上記のように、セル領域全体でROIを描画してください(図1)。
- データをカンマ区切り値 (CSV) ファイルにエクスポートして、後で分析します。
- または、無料で入手できるソフトウェアFIJI7 (https://fiji.sc)を使用して解析を実行します。
- 画像ファイルを開くには、 Bio-Formats プラグインを使用して、[ File] > [Import > Bio-Formats ] に移動し、ファイルを選択します。
- 次に、 虫眼鏡 ツールを使用してズームインし、 フリーハンド選択 ツールを使用して、応答セルの周囲に関心領域(ROI)を描画します。
- 各ROIを描画した後、 T キーを押して ROIマネージャーに追加します。対象のすべてのセルについて、このプロセスを繰り返します(図2)。
- すべてのROIを追加したら、 ROIマネージャー ウィンドウに移動し、すべてのROIを選択してから、メインメニューの 「分析」>「測定値の設定 」に移動します。
- 次に、ROI マネージャーに戻り、[ More > Multi Measure ] を選択して、すべてのフレームの強度を測定します。結果は新しいウィンドウに表示されます。CSVファイルとしてエクスポートするには 、[ファイル]>[名前を付けて保存]に移動します。
注意: CSVファイルは、Excel、Googleスプレッドシート、およびGraphPadソフトウェアで分析できます。式ΔF/F0 = (F(t) - F0)/F0 を使用して、Fluo-8 カルシウムフラックスアッセイキットで得られた蛍光強度値をカルシウム流入変化の相対的な測定値に変換します。ここで、F0 はベースライン蛍光を表し、カプサイシンによる刺激の前にt = 0 sからt = 60 sまで撮影された画像からの平均強度として計算されます。F(t)は、刺激後に観察された最大蛍光強度の値であり、これによりカルシウム流入のピークを評価できます。このアプローチは、ベースラインに対する蛍光の増加を強調しており、ΔF/F0 の上昇は細胞内カルシウムレベルの上昇を示します。

図1:LAS Xソフトウェアでのカルシウム流入解析のために選択した細胞のROIの例(A)定量モードのLAS Xインターフェース。ピンクの長方形は、定量化タブを示しています。黄色の四角形はポリラインの描画ツールを示し、シアン色の四角形はズームアウト ツールとズームイン ツールを示します。(B)キャプチャされた視野(FOV)。(C)FOVを拡大して、セル全体のROIの描画を容易にします。この図の拡大版を表示するには、ここをクリックしてください。
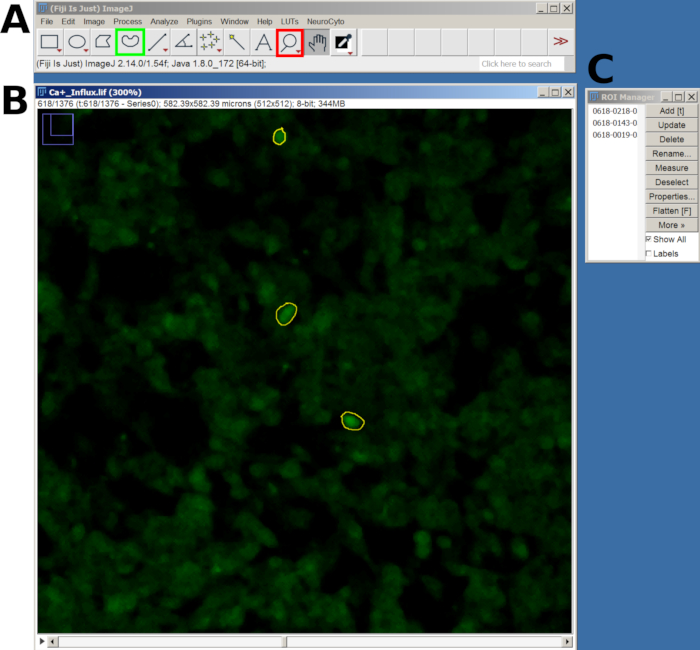
図2:FIJIソフトウェアでのカルシウム流入分析のために選択した細胞のROIの例(A)メインメニューとツールを示すFIJIインターフェース。赤い四角は虫眼鏡ツールを示し、緑の四角はフリーハンドの選択を示しています。(B)ズームインでキャプチャされた視野(FOV)と描画されたROI。(C) ROI マネージャー ウィンドウ。この図の拡大版を表示するには、ここをクリックしてください。
7. トラブルシューティング
- 画像のピントが合っていない場合は、次の操作を試してください。
- テープの接着性を確認してください。緩んでいると、針がずれてピントが合わなくなることがあります。
- 安定性を高めるために、追加のテープで針を再度取り付けます。
- 標準的な針の交換。プレート上のアライメントを改善するために、事前に曲げられた歯科用針先を使用します。
- 細胞が飽和したシグナルを示している場合は、次の手順を試してください。
- レーザー強度とゲイン設定を下げます。
- 試薬のインキュベーション時間を確認します。メーカーの推奨を超えると、信号が飽和状態になり、カルシウム流入の検出が複雑になる可能性があります。
- トラブルシューティングについては、次の追加の推奨事項に従ってください。
- 定期的なキャリブレーション:フォーカスと信号検出の品質を維持するために、イメージング機器が定期的にキャリブレーションされていることを確認してください。
- 変更の文書化: プロトコルに加えられた調整のログを、後で参照し、トラブルシューティングを容易にするために保持します。
- 事後対応型の調整:問題が解決しない場合は、異なる試薬濃度での試験や、シグナル検出の代替方法を検討してください。
結果
SH-SY5Y細胞が感覚様ニューロンに分化
ハイコンテントスクリーニング画像は、ニューロン分化のプロトコルがSH-SY5Y細胞の形態を変化させることを示しています。感覚様ニューロン細胞(分化細胞)は、ニューロフィラメントの広範なネットワークを投影する丸みを帯びた細胞体を示します。それらは、周囲のニューロンを接続するより細長い神経突起の?...
ディスカッション
侵害受容器は、痛みを媒介する感覚ニューロンの特殊なサブセットです。これらの細胞は、TRPV1などの電位依存性イオンチャネルやリガンドイオンチャネルを発現し、その活性化によりカルシウムの流入と、侵害受容伝達を調節する神経ペプチドや神経伝達物質の放出につながります。ここでは、SH-SY5Yを感覚様ニューロン細胞に分化させて、カプサイシン誘発性カル...
開示事項
MCB、AMCT、およびVOZは、変形性関節症の痛みに関与する分子実体を特定するプロセスに関する特許を所有しています(BR102018008561-1)。
謝辞
本研究は、Fundação Amparo à Pesquisa do Estado de São Paulo FAPESP Grant number 2015/50040-4 and 2020/13139-0, São Paulo Research Foundation and GlaxoSmithKline, FAPESP 2022/08417-7 and 2024/04023-0の支援を受けて行われました。
資料
| Name | Company | Catalog Number | Comments |
| All-trans retinoic acid | Tocris | 695 | |
| BDNF | Tocris | TOCR-2837 | |
| BDNF | Sigma-Aldrich | B3795 | |
| Butterfly type 23GA sterile | Beckton Dickinson Asepto | 38833814 | Scalp vein set |
| Capsaicin | Sigma-Aldrich | M2028 | |
| D-glucose | Sigma-Aldrich | G5767 | |
| DMEM/F12 | Gibco | 12500062 | Basal medium |
| D-ribose | Sigma-Aldrich | R7500 | |
| D-threose | Sigma-Aldrich | T7392 | |
| Fluo-8 Calcium Flux Assay Kit | Abcam | ab112129 | No wash |
| Heat-inactivated fetal bovine serum | Gibco | A5670801 | |
| High Content Screening | Molecular Devices | ||
| LASX software | Leica Microsystems | Microscopy software | |
| Leica TCS SP8 | Leica Microsystems | Leica TCS SP8 | Confocal microscope |
| Penicillin-streptomycin | Gibco | 15140130 | |
| Petri dish (35/10 mm) | Greiner bio-one | 627965 | |
| Rat tail type I collagen | Corning | 354236 | |
| SH-SY5Y | Merck | 94030304-1VL | Neuroblastoma cell line |
参考文献
- Bierhaus, A., et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 83 (11), 876-886 (2005).
- Hudson, B. I., et al. Blockade of receptor for advanced glycation endproducts: A new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys. 419 (1), 80-88 (2003).
- Dandia, H., Makkad, K., Tayalia, P. Glycated collagen - a 3D matrix system to study pathological cell behavior. Biomater Sci. 7 (8), 3480-3488 (2019).
- Bufalo, M. C., et al. Human sensory neuron-like cells and glycated collagen matrix as a model for the screening of analgesic compounds. Cells. 11 (2), 247 (2022).
- Frias, B., Merighi, A. Capsaicin, nociception and pain. Molecules. 21 (6), 797 (2016).
- Lam, D., et al. RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS One. 13 (2), e0193312 (2018).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Li, F., et al. TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun. 10 (1), 5678 (2019).
- Mickle, A. D., Shepherd, A. J., Mohapatra, D. P. Sensory TRP channels:the key transducers of nociception and pain. Prog Mol Biol Transl Sci. 131, 73-118 (2015).
- Iseppon, F., Linley, J. E., Wood, J. N. Calcium imaging for analgesic drug discovery. Neurobiol Pain. 11, 100083 (2022).
- Vetter, I., et al. Characterisation of Nav types endogenously expressed in human SH-SY5Y neuroblastoma cells. Biochem Pharmacol. 83 (11), 1562-1571 (2012).
- Dekker, L. V., et al. Analysis of human Nav1.8 expressed in SH-SY5Y neuroblastoma cells. Eur J Pharmacol. 528 (1-3), 52-58 (2005).
- Lam, P. M. W., Hainsworth, A. H., Smith, G. D., Owen, D. E., Davies, J., Lambert, D. G. Activation of recombinant human TRPV1 receptors expressed in SH-SY5Y human neuroblastoma cells increases [Ca2+]i initiates neurotransmitter release and promotes delayed cell death. J Neurochem. 102 (3), 801-811 (2007).
- Koplas, P. A., Rosenberg, R. L., Oxford, G. S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 17 (10), 3525-3537 (1997).
- Tominaga, M., et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21 (3), 531-543 (1998).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved