Method Article
双極性障害のラットモデルの開発
* これらの著者は同等に貢献しました
要約
この記事では、躁病様と抑うつ様の両方の行動を捉える双極性障害のユニークなラットモデルの誘導のためのプロトコルを紹介します。
要約
双極性障害は、感情的な高揚感(躁病)と低迷(うつ病)の期間を含む、極端な気分のむらを特徴とするメンタルヘルス状態です。その根底にある神経生物学の正確な解明はまだ完全には解明されていませんが、神経伝達物質系、特にドーパミンの不均衡が中心的な役割を果たしているようです。このため、ドーパミン作動性経路の操作は、げっ歯類の躁病またはうつ病をモデル化するために使用されてきました。ただし、これら 2 つのエピソード間の一般的な切り替えを正確に表すモデルはまれであり、顔の有効性が制限されています。ユニークなモデルでは、最新の技術を使用して、双極性障害の病理に関与しているドーパミンD1受容体の発現を一時的に増加させます。カルモジュリンキナーゼIIαプロモーターの制御下でドーパミンD1受容体を発現するテトラサイクリン誘導性レンチウイルスコンストラクトを成体ラットの内側前頭前皮質に定位的に注入します。ドーパミンD1受容体の過剰発現は、テトラサイクリン類似体であるドキシサイクリンを動物の飲料水に加えることで達成され、報酬関連、衝動的、リスクテイク行動の増加と不安の減少につながります。これらの行動は、躁病のような表現型に似ています。飲料水からドキシサイクリンを除去することにより、無力感の増加と無快感症を特徴とするうつ病様の表現型を同じ動物内で誘発することができます。.この記事では、手術を実施するための段階的なプロトコルと、双極性障害様表現型を誘発するための手順について説明します。さらに、躁病様および抑うつ様の行動に関連する行動変化を評価するための考慮事項について説明します。この有望なモデルは、優れた構成概念と顔の妥当性を示しており、双極性障害の病態生理学的メカニズムをさらに調査するための貴重なツールを提供します。
概要
双極性障害(BD)は、世界人口の約1%が罹患している重度の気分障害です1。それは、極端な気分、うつ病、躁病のエピソードと、ユーチミック状態によって特徴付けられます。BDのうつ病エピソードの症状は、単極性うつ病の症状と似ています。患者は、活動に対する関心と喜びの低下、悲しみ、絶望、無価値感を示します。さらに、食欲、睡眠行動、認知障害の変化がしばしば観察されます2。躁病エピソードは、異常に高まる気分、睡眠の必要性の低下、社会的脱抑制、自尊心の増加、誇大妄想、さらにはリスクテイクとイライラの高まりを特徴としています2。
BDの疾患の病因は、遺伝的要因と発生的要因の複雑な相互作用であるように思われますが3、その病態生理学に関与する正確なメカニズムはまだ完全には理解されていません。症状は神経伝達物質系の不均衡から生じると考えられており4 、特にドーパミン系に焦点を当てた研究は影響力があります5。例えば、Berkら6 は、ドーパミン仮説を仮定し、高ドーパミン作動性状態が躁病の根底にあり、うつ病は低ドーパミン作動症から生じると仮定しました。それ以来、動物モデルからの証拠、ならびに薬理学的および画像学的研究により、躁病症状と高ドーパミン痛症との関連について強力な支持が集まっています。また、ドーパミン作動性シグナル伝達の減少とうつ病エピソードとの関連が見つかる可能性がありましたが、程度は低いですが7。さらに、遺伝子調査の結果は、BD8のドーパミン仮説の考えを強化しています。
BDにおけるドーパミン系の役割をさらに解明するために、動物モデルを用いて症状の根底にある神経生物学的メカニズムを調査することができます。疾患モデルの適用と限界は、多くの場合、Willner9 が最初に提案した 3 つの検証基準に基づいて評価されます。これには、顔の妥当性、構成物の妥当性、および予測妥当性が含まれます。顔の妥当性は、障害の行動特性を模倣するモデルの能力を表します。障害の病態生理学と病因がモデルの基礎である場合、構成概念の妥当性は達成されますが、予測妥当性は、障害の薬理学的治療をモデル内で再現できることを意味します。
これまで、さまざまなげっ歯類モデルがBD10 の理解に貢献しており、広範囲の遺伝子改変、薬物的介入、および環境操作11が含まれています。
例えば、Clock遺伝子の実験的操作は、マウスにおいて躁病様の表現型を誘導することが示されている。転写因子CLOCKは概日リズムの調節に重要な役割を果たしており、Clock転写を活性化できないタンパク質を発現する遺伝子改変マウスは、多動性と報酬応答の増加を特徴とする12。結果として生じる表現型は、脳の腹側被蓋野におけるドーパミン作動性シグナル伝達のための異なる調節遺伝子によって媒介されているようである13。
精神刺激薬アンフェタミンなどのドーパミン増加薬の投与を通じてドーパミンシグナル伝達に直接影響を与えると、運動亢進を誘発することが示されており、その後の離脱は無快感症を含む抑うつ様症状に関連しています14。ケタミンまたはドーパミンD2/D3受容体アゴニストであるキンピロールによる薬理学的課題も、BD15,16に関連する行動を誘発することが示されています。
薬理学的介入に加えて、睡眠不足などの環境操作を使用して、BD17に関連する行動表現型を誘導することができます。睡眠不足の動物は、ドーパミンシグナル伝達の変化に関連する移動運動の増加と超音波発声の放出を特徴とする躁病様の表現型を示す18。
うつ病の19 または躁病のような20 の行動を研究するための他の多くのげっ歯類のモデルがあります。しかし、これらのモデルはすべてBDの病理の理解に大きく貢献していますが、一度に1つのエピソードしか研究していなかったり、短期的な影響しか研究していなかったりすると、限界があります。対照的に、感情状態間の特徴的な切り替えをモデル化することは困難でした。
ここでは、BDのユニークなラットモデルのプロトコルを示します。これは、ドーパミン系の単一の標的操作、すなわち、テトラサイクリン誘導性レンチウイルスコンストラクトから内側前頭前皮質(mPFC)におけるドーパミンD1受容体(DRD1)を条件付きで過剰発現することにより、1匹の動物で両方のエピソードを誘導することにより、顔面の有効性の向上を示しています。DRD1は、カルモジュリンキナーゼIIα(CamKIIa)プロモーターの制御下で遺伝子転写を駆動することにより、主にグルタミン酸作動性ニューロンで発現し、遺伝子操作の特異性を高めます。
元のレンチウイルス骨格pRRL.cPPT.WPRE.Sinは、Didier Trono博士(スイスのローザンヌ工科大学)21 によって提供され、GFPミニジーンをポリリンカー部位(レンチウイルスベクターPL13)に置き換えることによって改変されました。その後、PL13 を使用して PL13.pTRE2.DRD1.CamKIIa.rtTA3 または PL13.pTRE2.dsRedExpress.CamKIIa.rtTA3 を生成しました。ラットDRD1のcDNAはDavid Sibley博士(NINDS/NIH)22から、逆テトラサイクリン制御活性化因子3(rtTA3)cDNAはAtze Das博士とBen Berkhout博士(アムステルダム大学学術医療センター)から得られた23。CamKIIaプロモーターDNAはKarl Deisseroth博士(スタンフォード大学、カリフォルニア州)から提供され、dsRedExpressおよびテトラサイクリン応答要素2(pTRE2)配列は、それぞれ社内プラスミドpcDNA3.1-dsRedExpressおよびpcDNA3.1-pTRE2からサブクローニングされました。ウイルスベクターは、制限部位に隣接するPCR増幅DNA配列をサブクローニングすることによって作製しました。
このウイルスベクターを用いたモデルでは、mPFC CamKIIa陽性ニューロンにおけるDRD1の過剰発現が躁病様表現型24,25を誘発し、その後の遺伝子発現のダウンレギュレーションが抑うつ様行動を誘発することが示された26。疾患様表現型は1匹の動物で繰り返し誘導される可能性があるため27、モデルは高いレベルの顔の妥当性を反映している。さらに、ドーパミンシステムの操作は、DRD1レベル28,29またはDRD1多型の変化がBD病理30,31,32と関連しているため、BD7の動物モデルにとって強力な構成概念の妥当性を保持しています。
他の動物実験でも、前頭前野のDRD1の機能についての理解が深まっています。例えば、DRD1の減少はうつ病のモデルで一貫した発見であり33,34、一方、mPFCグルタミン酸作動性ニューロンにおけるDRD1の光遺伝学的刺激は不安を軽減し、抗うつ効果を誘発する35。Wu et al.36による最近の論文では、情動状態遷移におけるmPFC DRD1の役割が実証されています。この研究は、これらの受容体が興奮性シナプスの可塑性の根本的な変化に重要であることを強調しています。
全体として、mPFCのCamKIIa陽性ニューロンにおけるDRD1の標的操作と条件付き操作からなるBDのラットモデルを採用することは、高い構成概念と顔の妥当性を備えたモデルシステムを構成し、したがって、BDに関するトランスレーショナルリサーチの強力な可能性を示しています。
以下では、モデル生成のための外科的処置について説明します。さらに、モデル誘導と行動評価の方法論的考慮事項が、結果として生じる疾患様表現型の代表的な結果とともに提示されます。モデル生成と行動評価において考えられる障害と影響要因について議論し、将来の方向性についての見通しを示します。
プロトコル
ここに記載されている定位注射のプロトコルは、LANUV(Landesamt für Natur、Umwelt und Verbraucherschutz、Northrhine-Westfalia、Germany)によって承認されています。.成体の雄Sprague Dawleyラット(体重350-650g)を用いた。この研究で使用した試薬と機器は、 材料表に記載されています。
1. レンチウイルスコンストラクト
注:第3世代レンチウイルスシステムは、制御条件25,27としてDRD1または赤色蛍光タンパク質(dsRed)の条件付き発現に使用される。
- Stewartらによるプロトコル37 に基づいて、Addgeneリポジトリからのパッケージングプラスミド8454および8455を使用してレンチウイルスを産生します。
注:ウイルスの生産が独立して計画されていない場合、ドイツのシャリテベルリンなど、多くのコア施設が高力価レンチウイルスを提供しています。 - 力価を濃縮したウイルスを-80°Cで保存します。
- 注射用にμLあたり2×107 形質導入ユニット(TU)を調製します。
- ドライアイスでウイルスを手術室に運びます。
2. 動物たち
注:BDのラットモデルは、成体の雄Sprague Dawleyラット(体重350-650 g)で確立されています。雌ラットまたはそれ以前の発生時点の調査では、mPFCにおけるDRD1の発現が発生中に変化し、発情周期38,39,40の影響を受ける可能性があることを考慮することが重要です。
- ペアハウスラットと同条件の動物を、一定の温度と湿度の条件(相対湿度45%〜65%、温度22°C±2°C)で 食物 と水を自由に与えます。
- 行動調査は、暗闇での動物の活動期に実施する必要があるため、ラットを逆の12時間の明暗サイクル(午前11時に消灯)の下に保ちます。
- 実験を開始する前に、動物が施設と実験者の取り扱いに順応するために少なくとも7日間与えてください。
3.ウイルスコンストラクトの定位注射
注:安全フード(レンチウイルスを扱うための予防措置)および無菌状態で手術を行います。
- 準備
- 必要なすべての材料が利用可能で機能していることを確認します(材料の表)。
- 定位アームにシリンジホルダーを取り付けた状態で定位フレームをセットします。シリンジホルダーをシリンジポンプに接続します。
- デンタルドリルをセットアップし、0.9mmのバリを取り付けます。
- 加熱パッドを置き、37°Cに設定します。 ラットの位置決めを容易にするために、加熱パッドを適切な高さまで持ち上げます。
- 加熱パッドを吸収性ドレープで覆います。
- 無菌面にオートクレーブ滅菌した手術器具を調製します。
- 33 G注射針付きの10 μL定位固定薬注射シリンジをシリンジホルダーに取り付けます。
- 2.3 μLのウイルス懸濁液を、それぞれ1 μlの両側注射用に引き出します。引き出しが成功したことを視覚的に確認します。このステップは、レンチウイルスが室温になるまでの時間を最小限に抑えるための最後の準備ステップとして必ず実行してください。
- 鎮痛と麻酔導入
- 手術の朝、メロキシカム(1 mg / kg体重、私書箱)を投与します。.
- 鎮痛のための外科的処置の開始の20分前に、ラットにブプレノルフィン(0.5 mg / kg体重、皮下)を注射します。.
- 0.8-1 L / minの酸素流量で麻酔器の電源を入れます。
- 誘導チャンバーに4%イソフルランを浸水させ、ラットを誘導チャンバーに入れます。.
- 麻酔の導入が成功した後、呼吸の鈍化と意識の喪失によって見えるように、ラットを誘導室から取り出し、定位フレームに移動します。
- ラットの位置
- 麻酔の流れが鼻マスクに切り替えられていることを確認してください。
- ラットを誘導室から定位フレームに移し、前歯をホルダーに入れます。
- 麻酔マスクを鼻に適切に置き、イソフルランを1.5〜2.3%に回してメンテナンスします。.
- 滅菌アイクリームを使用して目を保護します。
- 切開を行う少なくとも10分前に、ラットにリドカイン(10 mg / kg体重、s.c.)を計画された切開部位の真下に局所的に注射します。.
- 耳棒を使用してラットを定位フレームに固定します。イヤーバーが均一で、頭の位置が水平であることを確認してください。
- 切開部位の周りの毛皮をハサミでトリミングします。皮膚消毒剤で湿らせたセルロースパッドを使用して毛皮を取り除きます。
- 皮膚消毒剤を使用して術野を消毒します。
- 開頭術とウイルスコンストラクトの注射
- つま先の反射がないことを確認して、適切な麻酔を確保します。
- 機器に触れる前に、手を消毒し、滅菌手袋に切り替えてください。
- メスの刃を使用して小さな内側切開(~1.5cm)を行います。
- ブルドッグクランプで皮膚を横に押し込むことにより、術野へのアクセスを確保します。
- 滅菌スワップを使用して、血液と残りの組織から術野を洗浄します。ブレグマの適切な視界と十分な前方スペースを確保します。
- A/P座標とM/L座標をブレグマに基づいてゼロに設定します。
- 定位アームを座標A/P+2.7、M/L±0.4まで動かし、消毒した鉛筆で可視化します。
- 直径 ~1 mm の穴を開け、両半球の射出側を覆います。
- 滅菌スワップを使用して血液を取り除きます。
- 脳の表面でD/V座標をゼロに設定し、注射針をゆっくりと-2.8まで下げて、mPFCの前縁領域に注入します。
- 組織が弛緩するまで5分間待ちます。
- 1 μL のウイルス懸濁液を 0.1 μL/分の速度で注入します。
- 針をゆっくりと取り外す前に、吸収のために5分待ちます。
- もう一方の半球で注入を繰り返します。
- 閉鎖と術後ケア
- 針を取り外し、骨ワックスを使用して頭蓋骨の開口部を閉じます。
- ブルドッグクランプを取り外し、皮膚を縫合します(3-0外科用縫合)。
- 術後鎮痛のためにラットにメロキシカム(1 mg / kg、皮下)を注射します。.
- 麻酔をオフにし、動物を定位フレームから取り出し、ホームケージに入れます。ネズミが完全に目を覚ますことを確認してください。
- シリンジを100%エタノールですすいで残りのレンチウイルスを不活性化し、続いて次の注射に備えて蒸留H2Oを注入します。
- メロキシカム (1 mg/kg, p.o.) による術後鎮痛を 24 時間ごとに 3 日間にわたって行い、動物の健康状態を 1 週間採点します。
- 手術後最初の24時間は、他の人が縫合糸を改ざんするのを防ぐために、一匹の家で動物を飼います。その後、ケージメイトと一緒に戻します。
4. モデル導入のためのドキシサイクリン治療
注:注入後24時間でモデル導入を開始します。また、注射から導入までの間に最大数か月の長い期間待つこともできます(たとえば、より大きな動物のコホートを同時にテストするため)。これは、ウイルスコンストラクトの機能に影響を与えないことが示されています。
- マニアっぽいエピソードの誘導
- 躁病様の表現型を誘発するには、動物に0.5 g / Lのドキシサイクリンヒクレートを飲料水に加えて与えます。.これにより、ウイルスの転写と追加のDRD1の過剰発現が誘導されます。
- ドキシサイクリン含有水を48時間〜72時間ごとに新たに調製するが、これは、不透明な水筒41であっても、ドキシサイクリンの安定性が影響を受けない期間である。
注:ドキシサイクリンを7日間治療した後、ウイルス媒介性過剰発現が最大に達し、 ?? 病のようなエピソード中に行動調査を行うことができます。.
- うつ病様エピソードの誘発
- 動物を通常の飲料水に戻し、うつ病のようなエピソードを誘発します。
- ウイルスの転写が停止するまで4日間待ってから、うつ病様エピソードの行動評価を行います。.
- 同じパターンに従って、後続のエピソード誘導を実行します。
5. 行動評価
注:モデル誘導に続いて、バイポーラ様の振る舞いを評価できます。臨床症状からげっ歯類で観察可能な行動パターンへの翻訳のさまざまなフレームワークが提案されています。最も影響力のあるものの1つは、研究領域基準42であり、精神障害に影響を受ける可能性のある機能と行動の領域の変化が調査されます。しかし、種の障壁のために、げっ歯類では自殺傾向などの一部の症状を調査することができないことに注意することが重要です43。ラットモデルは、その高度な認知能力と感情能力により、トランスレーショナル症状評価44の特に高い可能性を秘めており、より精巧なテスト手順を可能にします。行動評価に関する考慮事項を 表 1 に示します。
- 行動テスト45の一連のものとして行動調査を計画し、結果として生じる表現型の全体像を提供する。
- より侵襲的なテストを最後に行うように注意してください。
- 両方の疾患様エピソードで1匹の動物を試験しようとする場合、以前の試験経験から生じる可能性のある行動の変化を考慮してください。
注:手元の質問に応じて、躁病様またはうつ病様のエピソード中にラットの異なるグループをテストすることは有益であり、これにより、それぞれのエピソード中に組織を収集することも可能になります。経験上、行動にナイーブな動物を試験すると、特定の行動試験でより顕著な表現型が得られることが示されています。 - また、住居条件46 または実験者47の性別などの他の要因も考慮に入れる。
- 動物福祉のためだけでなく、精神医学的表現型とストレスとの相互作用の可能性を排除するためにも、動物への意図しないストレスを減らすために、あらゆる可能な手段を採用すること48。
- 概日リズムの乱れはBD17の症状であるため、概日リズムに特に注意してください。ネズミは夕暮れと夜明けに最も活動的であるため、逆明暗サイクル49の下で飼育されている動物で薄暗い赤色光の下でテストする必要があります。
注: ここで示す代表的な結果のほとんどは、このアプローチに従って収集されています。しかし、動物たちの昼夜のサイクルを切り替えずに行動評価を行えば、病気のような表現型は依然として観察可能である27。 - 実験は常に事前登録し、PREPARE50 およびARRIVE51 のガイドラインに従って実施および説明してください。
結果
ドキシサイクリンを動物の飲料水に加えると、追加のDRD1が発現し、7日後には、動物に躁病様行動をテストするのに十分な過剰発現が見られます。これまでのところ、報酬関連の行動の増加が示されています。躁病のような動物は、対照と比較して、2本の選択テストで水に対してより多くのショ糖溶液を飲みます25。受容力のある女性と一緒に観察ボックスに入れられ、25分間観察すると、躁病様動物は対照と比較してより多くの性的マウントを示します27 (図1A)。コカインの自己投与パラダイムでは、彼らは固定比率のスケジュールでより多くのコカインを投与し、プログレッシブ比率のスケジュールではより高いブレークポイントを示します。彼らの用量反応曲線は、低用量に対するより高い感度にシフトしています25。この感度の変化は、いくつかの場所のコンディショニングパラダイムにおける動機付けの顕著性の増加にも見られます。躁病のような動物は、対照群と比較して、ニコチン、アルコール、およびコカインの条件付けされた側でより多くの時間を過ごしました25。遅延割引に関するT迷路ベースのテストでは、新規性を求めることの増加と衝動的な選択の増加も見られた25。アイオワギャンブルタスクのオペラントラットバージョンでは、躁病のような動物は、対照24 (図1B)と比較して、不利な(高リスク、高利得)選択をより頻繁に決定します。躁病のような動物の不安は、高架のプラス迷路25で両手を広げて過ごす時間が増えることによって示されるように、減少します。
うつ病様の表現型は、DRD1の過剰発現を終わらせることによって誘発され得る。うつ病様エピソードでは、無力感の増加が観察される可能性があります。無力感のトライアドパラダイムでは、初めて電気ショックを受けたグループ(図1C)と、ショックの制御を学んだグループは、それぞれのコントロールと比較して、脱出遅延が増加してより無力でした27。無力感が誘発された群では、実験動物と対照動物との間に差は見られなかった。無快感症は、ショ糖27 の2本ボトル選択テストと性行動(未発表データ)で発見されました。大理石の埋設試験では、うつ状態の動物もより不安であった26 (図1D)。
記載された動物モデルは、躁病またはうつ病様の行動を調査する可能性を提供するだけでなく、DRD1の過剰発現を終了する際の行動の切り替えを観察するユニークな機会も提供します。これは、患者の躁病からうつ病への切り替えに似ています。ここでは、特定の行動に慣れることを念頭に置き、最小限の慣れでテストを選択することが重要です。例えば、躁病様エピソードおよび抑うつ様エピソードにおける性行動の増加は、対照動物に見られるようなレベルまでそのような行動の減少を示した。この実験では、同じ動物内で3つの躁病/抑うつ様サイクルが誘発された27。ショ糖飲用の場合、躁病様状態でのショ糖溶液の嗜好性は、うつ病様状態に切り替えると制御レベルまで低下しただけでなく、減少しました27。アイオワ州のギャンブル課題のラットバージョンでは、不利な選択肢の数は躁病様動物で増加しましたが、動物がうつ病様状態の対照群と有意差はありませんでした。後者の状態では、全体の稼ぎ餌の数は、対照動物24と比較して減少した。
全体として、動物は堅牢な双極性表現型を示し、両方のエピソードで異なる行動領域で観察できます。このモデルでは、エピソード間の切り替えが顔の有効性の向上に貢献しています。影響を受ける行動ドメインの概要を 図2に示します。
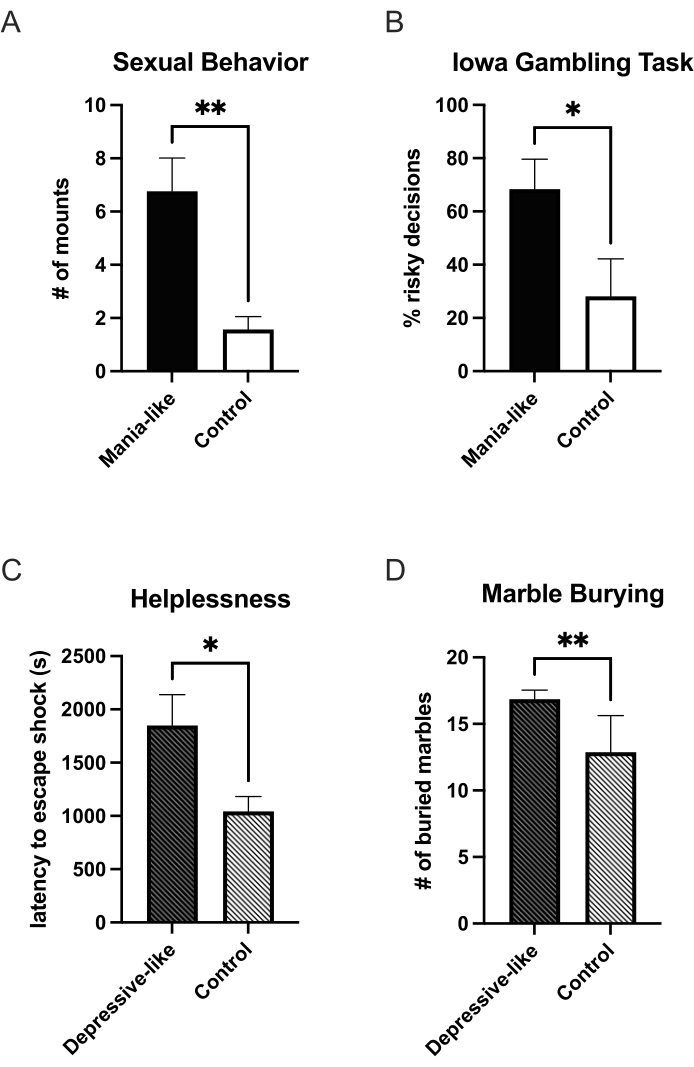
図1:ウイルスDRD1の過剰発現後の躁病様およびうつ病様状態の行動変化。 ウイルス性DRD1の過剰発現中、躁病様の状態では、動物は対照群と比較して、より多くの性的マウント(A)を示し、アイオワギャンブルタスク(B)で危険な選択が増加します。過剰発現の終了後、動物は鬱病様の状態に切り替わります。彼らは無力感(C)と不安(D)の増加を示しています。*p < 0.05;**p < 0.01;エラーバーは、平均の標準誤差を示します。 この図の拡大版を表示するには、ここをクリックしてください。
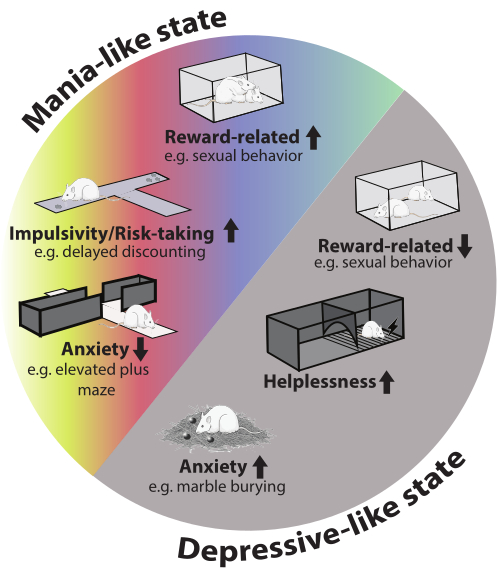
図2:モデルの行動表現型。 躁病様エピソードでは、報酬関連の行動(性行動など)、衝動性、リスクテイクが増加します。高架プラス迷路テストでは不安が軽減されました。うつ病様エピソードでは、ビー玉埋めテストで不安が増大し、性行動が減少し、動物はより多くの無力感を示しました。図のネズミの画像はServier Medical Artから取得され、CC BY 4.0の下でライセンスされています。 この図の拡大版を表示するには、ここをクリックしてください。
表 1: 行動評価に関する考慮事項。 この表は、行動評価中の主な実験ステップに関する重要な考慮事項を示しています。 この表をダウンロードするには、ここをクリックしてください。
ディスカッション
ここでは、顔の有効性が向上したBDの新しいラットモデルが提示されます。mPFCにおけるDRD1の標的操作により、同じ動物に躁病様およびうつ病様の表現型を誘導することができます。代表的な結果は、両方のエピソードで観察可能な疾患様表現型を強調しています。このモデルは比較的簡単に適用できます。DRD1またはdsRedのいずれかをコントロールとして発現する2つの誘導性レンチウイルスベクターが必要です。動物でのレンチウイルスシステムの製造および使用には、一定の安全性レベルが必要であり、それを実施する必要があります。ウイルス製造に必要な設備が利用できない場合、中核施設での作業経験はプラスです。
モデルを生成するための最も重要なステップは、レンチウイルスシステムの定位注射です。定位手術は神経科学において確立された手順であり、訓練を受けた研究者の間で成功率は高いです。エラーの原因としては、主に 2 つ考えられます。麻酔の問題は、外科的処置中に死亡につながる可能性があります。ここでは、プロトコルに記載されているように、イソフルラン吸入麻酔を使用することが、簡単に調整可能な薬物レベルが注射麻酔と比較して明らかな利点を構成するため、最良のアプローチであることが証明されています。イソフルランは鎮痛効果をもたらさず、髄膜侵害受容器は刺激に敏感であるため52、術中鎮痛にはオピオイドを使用することをお勧めします。プロトコルに記載されているように、適切な術後投薬と組み合わせると、術後の痛みの観察可能な兆候はありません。しかし、研究課題に対する可能性のある影響、例えば、ドーパミンとオピオイド系との相互作用に関しては、常に考慮されるべきであり、それに応じて適切な投薬計画が選択されるべきである53。手術が無菌条件下で行われる場合、感染症の発生や創傷治癒の障害はまれです。手術中または回復中に問題が発生した場合、トラブルシューティングは、説明されているプロトコルの正しい実行に焦点を当てる必要があります。無菌作業条件を確保し、薬剤の正確な投与を行うことが不可欠です。液体またはグルコース溶液の投与は、さらに回復を助けることができます。感染が発生した場合、テトラサイクリンはウイルスシステムの転写と相互作用するため、治療にはテトラサイクリンを含めないでください。.術後創傷感染症の第一選択治療は、エンロフロキサシンであり、おそらくカルプロフェンと併用されます。
手術中のエラーの別の考えられる原因は、注射を標的領域の外側に配置することです。しかし、プロトコルが正しく守られ、動物の頭部の適切な位置が確保されている場合、これはめったに起こりません。配置が成功したかどうかは、常に確認する必要があります。dsRed発現ウイルスの配置は対照動物で容易に検出できますが、DRD1発現ウイルスの配置検証には追加の手順が必要です。ウイルスコンストラクトのさまざまな部分に対して抗体染色を行っても、満足のいく結果は得られませんでした。Beyer et al.24に記載されているように、mPFCを解剖し、PCRを実施してrtTA3転写産物を検出することにより、ウイルスの配置を確認することをお勧めします。また、ウイルスの注射は、同量のウイルスと二国間で行う必要があることに注意することも重要です。脳と行動の側方化は、双極性障害54,55の患者で異なることが示されており、片側のウイルス注射は望ましい行動表現型を誘発しない可能性があります。
ウイルスのDRD1発現の誘導と、飲料水にドキシサイクリンを添加することによる躁病様エピソードは非常にうまく機能します。通常の飲料水をドキシサイクリンで置き換えても、飲酒行動に顕著な変化を引き起こさないことが証明されています。.ただし、液体の消費量を監視する必要があります。他の物質、例えば、薬物が飲料水 を介して 投与されることを意図している場合、調節が可能です。ドキシサイクリン投与は、食品ペレット を介して も行うことができます。.しかし、これはまだ検証されていません。
行動調査については、いくつかの考慮事項を 表1に示します。特に、実験を計画する際には、モデル固有の要件を評価する必要があります。例えば、2つのグループの動物を試験するかどうか、または1つの動物が両方のエピソードで行動評価を受けるかどうかを決定する必要があります。そのため、再試験が必要になるかもしれません。行動調査中に双極性様表現型が検出されない場合、配置は確認できますが、トラブルシューティングは行動結果に影響を与える可能性のあるさまざまな要因に焦点を当てることができます。実験者や概日リズムの変化は、環境内のストレスの多い条件が行動結果に影響を与える可能性があるため、プロセス中に批判的に評価する必要があります。
モデルは良好な構成体と顔の妥当性を示していますが、予測的妥当性はまだ評価する必要があります。BD56の第一選択治療としての慢性リチウム投与は、モデルによる行動の変化を防ぐのに成功するはずです。BDで使用される他の薬物(抗精神病薬や抗けいれん薬など)に対する反応を調査して、モデルの予測的妥当性を完全にテストすることができます。
さらに、現在の限界は、雌動物におけるモデルの有効性を将来の研究で評価する必要があることです。前臨床研究に雌動物を含める傾向にある一方で、これはまだ無視されがちです。提示されたモデルでは、ドーパミン系と発情周期との相互作用が予想されます。しかし、どの程度発生するかは不明です。また、精神医学動物モデルの一般的な制限を念頭に置くことも重要です。1匹のラットで両方の疾患様エピソードを誘発する可能性は、顔の妥当性を高めますが、外部から誘発される変化は、BD患者における疾患エピソードの自然発生および循環とは依然として異なります。このモデルはドーパミン系の標的操作のみに基づいているため、ドーパミン伝達の変化と関連する二次的影響によって主要な影響が引き起こされます。したがって、BDの症状に対する他のシステムの寄与は考慮されていません。
結論として、提示されたモデルは、両方の疾患エピソードを1匹の動物で研究できるため、BDを調査するための強い可能性を秘めています。これは、ほとんどの確立されたモデルと比較して、エピソード間の遷移を調査するためのユニークな可能性を示しています。提示されたプロトコルには、ほとんどの前臨床研究所で利用可能な機器と技術スキルが必要であり、広く適用できます。これまでのところ、結果として得られる行動表現型は、さまざまな行動にわたって堅牢です。社会的行動57 や認知機能などの他の領域はまだ探求されていません。提示されたプロトコルは行動の結果に焦点を当てていましたが、分子メカニズムをさらに調査するための将来のアプリケーションにはさまざまな可能性があります。BDの病因の根底にあるメカニズム、特にエピソード間の移行を理解するために研究を拡大することで、最終的に将来の臨床応用に変換できる可能性のある治療標的の特定につながる可能性があります。
開示事項
著者は何も開示していません。
謝辞
この研究は、ドイツ研究財団(Deutsche Forschungsgemeinschaft、DFG)からの助成金によって支援されました:プロジェクト番号552842155およびGRK2862/1、プロジェクト番号:492434978。JAは、ルール大学ボーフム医学部のFoRUM研究基金(助成金番号P109-24)から資金提供を受けました。図2のネズミの画像はServier Medical Artから取得され、CC BY 4.0でライセンスされています。
資料
| Name | Company | Catalog Number | Comments |
| 0.9 mm burr | FST | 19007-09 | Burr for craniotomy |
| 10 µl Neuros Syringe | Hamilton | 65460-06 | Mounted to syringe pump for injection |
| 1ml single use Syringes | Braum | 9166017V | Administration of medication |
| 33 G Needles | Hamilton | 65461-02 | Replacement needles for neuros syringe |
| 4-way valve | UNO | 180000259 | For simultaneous connection of induction chamber and face mask |
| Absorbent Drape | Sabanindas | 1834014 | Covering equipment before placing the animal |
| Anaesthetic Gas Filter | UNO | 180000140 | Anesthesia fume collection |
| Anasthesia mask for stereotactic | Hugo Sachs Electronic | 73-4922 | Administering anesthesia during surgery |
| Anesthesia vaporiser | UNO | 180000002 | Provide and adjust levels of vaporised isoflurane |
| Bone wax | SMI | Z046 | Closing the hole in the skull |
| Bulldog clamps | FST | 18038-45 | To retain skin and allow access to the surgical field |
| Buprenorphine | Elanco | 18760711 | Interoperative analgesia |
| Cannula | Tegler | T138339 | Administration of medication |
| Cellulose swabs | Meditrade | 1177 | Cleaning Skin |
| Connector | UNO | 180000005 | Connecting anesthesia tubing to face mask |
| Control Unit for heating pad | UNO | 180000122 | Controlling heating pad |
| Dental Dril | Saeyang | SMT K-38 | Dental drill for craniotomy; equipable with fine dental burrs |
| Desktop digital stereotaxic instrument | RWD | E03135-002 | Fully equipped stereotactic frame with digital manipulator |
| Destilled H2O | - | - | Rinsing the syringe |
| Doxycycline hyclate | Sigma aldrich | D9891 | For model induction |
| Dry ice | - | - | Transporting viral suspension |
| Earbars | RWD | 68302 | Head fixation in the stereotactic frame |
| Ethanol | - | - | Rinsing the syringe and deactivating virus |
| Flowmeter | UNO | CM2 | Verify and adjust flow rate |
| Forceps - anatomical | FST | 11000-12 | Holding skin |
| Forceps - surgical | FST | 11027-12 | Holding skin |
| Heating pad | UNO | 180000028 | Heating pad for keeping the animal warm during surgery |
| Induction chamber | UNO | 180000233 | Chamber for initial induction of anesthesia |
| Isoflurane | CP Pharma | V7005232.00.00 | Anesthesia |
| Lentiviral suspension | - | - | Lentiviral construct coding for DRD1 or dsRed for model induction |
| Lidocaine | Combustin | 8780701 | Local analgesia |
| Meloxicam | Boehringer Ingelheim | 7578423 | Pre- and postoperative analgesia |
| Needle holder | FST | 91201-13 | Sutering |
| Oxygen concentrator | UNO | 180000399 | Providing oxygen for anesthesia |
| PE Tubing | - | - | Connecting components of the anesthesia machine to induction chamber & face mask |
| Pencil | - | - | Marking the correct side for craniotomy |
| Scalpel blade holder | FST | 10003-12 | To hold scalpel blade |
| Scapel blades | FST | 10011-00 | Fine surgical blade for incision |
| Scavenger Unit | UNO | 180000260 | Controlling capacity of fume collector |
| Skin disinfectant | Bode | 975042 | Disinfacting skin before incision |
| Sterile cotton swabs | Boettger | 1102241 | Cleaning surgical field |
| Sterile eye cream | Bayer | 1578675 | Protect eyes during surgery |
| Surgical Scissors | FST | 14000-12 | Trimming fur and cutting suture material |
| Suture 3-0 polyglycolic acid | SMI | 11201519 | Suturing skin |
| Syringe pump | KdScientific | 788130 | Syring pump with connectable holder |
参考文献
- Müller-Oerlinghausen, B., Berghöfer, A., Bauer, M. Bipolar disorder. Lancet. 359 (9302), 241-247 (2002).
- Grande, I., Berk, M., Birmaher, B., Vieta, E. Bipolar disorder. Lancet. 387 (10027), 1561-1572 (2016).
- Vieta, E., et al. Bipolar Disorders. Nat Rev Dis Primers. 4 (1), 1-16 (2018).
- Lee, J. G., et al. Neuromolecular etiology of bipolar disorder: Possible therapeutic targets of mood stabilizers. Clin Psychopharmacol Neurosci. 20 (2), 228-239 (2022).
- Mohamadian, M., et al. Mood and behavior regulation: Interaction of lithium and dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 396 (7), 1339-1359 (2023).
- Berk, M., et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand. 116 (s434), 41-49 (2007).
- Ashok, A. H., et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 22 (5), 666-679 (2017).
- Zhang, C. -. Y., et al. Genetic evidence for the "dopamine hypothesis of bipolar disorder.". Mol Psychiatry. 28 (2), 532-535 (2023).
- Willner, P. The validity of animal models of depression. Psychopharmacology. 83 (1), 1-16 (1984).
- Beyer, D. K. E., Freund, N. Animal models for bipolar disorder: From bedside to the cage. Int J Bipolar Disord. 5 (1), 35 (2017).
- Valvassori, S. S., Gava, F. F., Cararo, J. H., Quevedo, J. Chapter 9 - The evolution of animal models for bipolar disorder. Neurobiol Bipol Dis. , 109-115 (2021).
- McClung, C. A., et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 102 (26), 9377-9381 (2005).
- Roybal, K., et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 104 (15), 6406-6411 (2007).
- Pathak, G., Ibrahim, B. A., McCarthy, S. A., Baker, K., Kelly, M. P. Amphetamine sensitization in mice is sufficient to produce both manic- and depressive-related behaviors as well as changes in the functional connectivity of corticolimbic structures. Neuropharmacol. 95, 434-447 (2015).
- Krug, J. T., et al. Effects of chronic lithium exposure in a modified rodent ketamine-induced hyperactivity model of mania. Pharmacol Biochem Behav. 179, 150-155 (2019).
- Shaldubina, A., Einat, H., Szechtman, H., Shimon, H., Belmaker, R. H. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J Neural Transm. 109 (3), 433-440 (2002).
- Freund, N., Haussleiter, I. Bipolar chronobiology in men and mice: A Narrative review. Brain Sci. 13 (5), 738 (2023).
- Wendler, E., et al. Mania-like elevated mood in rats: Enhanced 50-kHz ultrasonic vocalizations after sleep deprivation. Prog Neuropsychopharmacol Biol Psychiatry. 88, 142-150 (2019).
- Krishnan, V., Nestler, E. J. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 7, 121-147 (2011).
- Schmerder, K., Freund, N. Animal models for mania. Psychiatr Vulnerab Mood Anxiety Disord. 190, 233-277 (2023).
- Zufferey, R., et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 72 (12), 9873-9880 (1998).
- Gardner, B., Liu, Z. F., Jiang, D., Sibley, D. R. The role of phosphorylation/dephosphorylation in agonist-induced desensitization of D1 dopamine receptor function: Evidence for a novel pathway for receptor dephosphorylation. Mol Pharmacol. 59 (2), 310-321 (2001).
- Das, A. T., et al. Viral evolution as a tool to improve the tetracycline-regulated gene expression system. J Biol Chem. 279 (18), 18776-18782 (2004).
- Beyer, D. K. E., Horn, L., Klinker, N., Freund, N. Risky decision-making following prefrontal D1 receptor manipulation. Transl Neurosci. 12 (1), 432-443 (2021).
- Sonntag, K. C., et al. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: Comparison with adolescents. Psychopharmacology. 231 (8), 1615-1626 (2014).
- Beyer, D. K. E., Mattukat, A., Freund, N. Prefrontal dopamine D1 receptor manipulation influences anxiety behavior and induces neuroinflammation within the hippocampus. Int J Bipolar Disord. 9 (1), 9 (2021).
- Freund, N., Thompson, B. S., Sonntag, K., Meda, S., Andersen, S. L. When the party is over: Depressive-like states in rats following termination of cortical D1 receptor overexpression. Psychopharmacology. 233 (7), 1191-1201 (2016).
- Pantazopoulos, H., Stone, D., Walsh, J., Benes, F. M. Differences in the cellular distribution of D1 receptor mRNA in the hippocampus of bipolars and schizophrenics. Synapse. 54 (3), 147-155 (2004).
- Suhara, T., et al. D1 dopamine receptor binding in mood disorders measured by positron emission tomography. Psychopharmacology. 106 (1), 14-18 (1992).
- Dmitrzak-Weglarz, M., et al. Dopamine receptor D1 Gene -48A/G polymorphism is associated with bipolar illness but not with schizophrenia in a polish population. Neuropsychobiology. 53 (1), 46-50 (2006).
- Rybakowski, J., Dmitrzak-Weglarz, M., Suwalska, A., Leszczynska-Rodziewicz, A., Hauser, J. Dopamine D1 receptor gene polymorphism is associated with prophylactic lithium response in bipolar disorder. Pharmacopsychiatry. 42 (01), 20-22 (2009).
- Severino, G., et al. A48G polymorphism in the D 1 receptor genes associated with bipolar I disorder. Am J Med Genet B Neuropsychiatr Genet. 134B (1), 37-38 (2005).
- Shinohara, R., et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry. 23 (8), 1717-1730 (2018).
- Yang, Y., Zhong, Z., Wang, B., Wang, Y., Ding, W. Activation of D1R signaling in the medial prefrontal cortex rescues maternal separation-induced behavioral deficits through restoration of excitatory neurotransmission. Behav Brain Res. 441, 114287 (2023).
- Hare, B. D., et al. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 10 (1), 223 (2019).
- Wu, M., et al. Dopamine pathways mediating affective state transitions after sleep loss. Neuron. 112 (1), 141-154 (2024).
- Stewart, S. A., et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 9 (4), 493-501 (2003).
- Andersen, S. L., Thompson, A. T., Rutstein, M., Hostetter, J. C., Teicher, M. H. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 37 (2), 167-169 (2000).
- Brenhouse, H. C., Sonntag, K. C., Andersen, S. L. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: Relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 28 (10), 2375-2382 (2008).
- Thompson, T. L., Moss, R. L. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 229 (3), 145-148 (1997).
- Redelsperger, I. M., et al. Stability of doxycycline in feed and water and minimal effective doses in tetracycline-inducible systems. J Am Assoc Lab Anim Sci. 55 (4), 467-474 (2016).
- Insel, T., et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 167 (7), 748-751 (2010).
- von Mücke-Heim, I. -. A., et al. Introducing a depression-like syndrome for translational neuropsychiatry: a plea for taxonomical validity and improved comparability between humans and mice. Mol Psychiatry. 28 (1), 329-340 (2023).
- Ben-Ami Bartal, I. The complex affective and cognitive capacities of rats. Science. 385 (6715), 1298-1305 (2024).
- Jaehne, E. J., Corrone, M., van den Buuse, M. Administering a behavioral test battery in rodents. Neurobiol Methods Protoc. , 87-100 (2024).
- Prager, E. M., Bergstrom, H. C., Grunberg, N. E., Johnson, L. R. The Importance of reporting housing and husbandry in rat research. Front Behav Neurosci. 5, 38 (2011).
- Sorge, R. E., et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 11 (6), 629-632 (2014).
- Du Preez, A., et al. Do different types of stress differentially alter behavioral and neurobiological outcomes associated with depression in rodent models? A systematic review. Front Neuroendocrinol. 61, 100896 (2021).
- Burn, C. C. What is it like to be a rat? Rat sensory perception and its implications for experimental design and rat welfare. Appl Anim Behav Sci. 112 (1), 1-32 (2008).
- Smith, A. J., Clutton, R. E., Lilley, E., Hansen, K. E. PREPARE: Guidelines for planning animal research and testing. Lab Anim. 52 (2), 135-141 (2018).
- Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8 (6), e1000412 (2010).
- Messlinger, K., Ellrich, J. Meningeal nociception: Electrophysiological studies related to headache and referred pain. Microsc Res Tech. 53 (2), 129-137 (2001).
- Jirkof, P. Side effects of pain and analgesia in animal experimentation. Lab Anim. 46 (4), 123-128 (2017).
- Moebus, L., Quirin, M., Ehrlenspiel, F. Cerebral asymmetry in bipolar disorders: A scoping review. Biol Psychol. 179, 108551 (2023).
- Mundorf, A., Borawski, J., Ocklenburg, S. Behavioral lateralization in bipolar disorders: A systematic review. Int J Bipolar Disord. 11 (1), 37 (2023).
- Alda, M. Lithium in the treatment of bipolar disorder: Pharmacology and pharmacogenetics. Mol Psychiatry. 20 (6), 661-670 (2015).
- Reinhardt, P. R., Theis, C. D. C., Juckel, G., Freund, N. Rodent models for mood disorders - understanding molecular changes by investigating social behavior. Biol Chem. 404 (10), 939-950 (2023).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved