室温以下の反応を実施
概要
博士ダナ ラシュリー - 大学のウィリアムとメアリーのソース: 研究室
デモンストレーション: マット ・ スミス
化学反応の過程で新たな結合が形成される関係種 (原子または分子) 非常に近くに来るし、互いに衝突することが必要です。これらの種の衝突がより頻繁にかつ効果的なより高い速度では、これらの分子が移動します。アレニウス方程式1、約、10 K で温度を上げ状態にそのルーツを持って広く使用されているルールの親指、反応の速度を 2 倍し、20 K で温度を上げ、速度を 4 倍増します。
(1)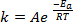
式 (1) しばしばその対数であります。
(2)
どこkは化学反応の速度、 (分子衝突の頻度に関連する) 頻度因子は、 E、反応に必要な活性化エネルギー、 Rは理想的な気体定数、 Tは温度で反応が行われています。
高い温度したがって、反応がはるかに速く完了を意味します。それにもかかわらず、いくつかのケースで、反応率の低下の効果にもかかわらず、低温で反応を行うことをお勧めします。いくつかのシナリオがこの点詳しくは、さらに以下。
それが室温以下の反応を実行するのに役に、化学者は特定の温度または温度範囲を維持するために冷却槽を使用します。反応は、適切な冷却槽内反応フラスコを配置することによって所望の温度に冷却です。反応試薬は、冷却槽での化学物質との直接接触してこない。冷却槽 (氷、ドライアイス、液体窒素など) 1 つのクライオ (冷却) コンポーネントから成るかもしれないまたは特定の溶媒および添加物塩と低温の成分の混合物があります。溶媒の目的は反応フラスコに冷却剤の温度を効果的に転送して、添加剤の目的は、混合物の凍結ポイントを下げる (または抑制)。(溶剤と添加剤をする物質のことであるに注意してください)。
手順
冷却バス セットアップ
一般的なセットアップのため以下のとおり選択の冷却槽の準備、風呂 (図 1) に反応フラスコを浸します。すべての方法で、風呂の容器を記入しないが、反応フラスコの液浸のため許可する十分な部屋を去る。
注: 反応が湿気敏感な場合は、試薬をフラスコまたは (例えば滴下漏斗) 装置の他の部分に追加する場合非常に注意してください。ガラスが冷却浴に浸漬しながら開口部が生成される場合は、常温空気はすぐに中に流れてし、水分を 。

図 1。冷却バス セットアップには滴下漏斗で三首丸底フラスコ、不活性雰囲気下で温度計の例。
1. 氷水浴を作る
申請書と概要
参考文献
- Gordon, A. J., Ford, R. A. The Chemist's Companion - A Handbook of Practical Data, Techniques, and References. Chapter 11 (1973) ISBN: 978-0-471-31590-2.
- Rondeau, R. E. Slush baths. J. Chem. Eng. Data. 11, 124 (1966)
スキップ先...
このコレクションのビデオ:

Now Playing
室温以下の反応を実施
Organic Chemistry
70.6K 閲覧数

触媒入門
Organic Chemistry
34.5K 閲覧数

温水の化学反応のための還流システムの組み立て
Organic Chemistry
167.4K 閲覧数

Schlenk ライン溶剤伝
Organic Chemistry
41.6K 閲覧数

凍害ポンプ サイクリングで液体の脱気
Organic Chemistry
56.1K 閲覧数

無水試薬と機器の準備
Organic Chemistry
79.3K 閲覧数

再結晶により物質を浄化
Organic Chemistry
708.5K 閲覧数

沈殿物によって混合物の分離
Organic Chemistry
157.8K 閲覧数

固液抽出
Organic Chemistry
237.8K 閲覧数

溶媒を除去する回転蒸発
Organic Chemistry
212.8K 閲覧数

分別蒸留
Organic Chemistry
334.3K 閲覧数

X 線回折用結晶を成長
Organic Chemistry
32.4K 閲覧数

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.7K 閲覧数

カラム ・ クロマトグラフィ
Organic Chemistry
360.1K 閲覧数

核磁気共鳴 (NMR) 分光法
Organic Chemistry
247.8K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved