Durchführung von Reaktionen unterhalb der Raumtemperatur
Überblick
Quelle: Labor von Dr. Dana Lashley - College of William and Mary
Demonstration von: Matt Smith
Wenn neue Anleihen im Verlauf einer chemischen Reaktion entstehen, erfordert es, dass die beteiligten Spezies (Atome oder Moleküle) in unmittelbarer Nähe kommen und miteinander kollidieren. Die Zusammenstöße zwischen diesen Arten sind häufiger und effektiver je höher die Geschwindigkeit mit dem diese Moleküle bewegen. Eine weit verbreitete Faustregel, hat seine Wurzeln in der Arrhenius Gleichung1, Staaten, die Erhöhung der Temperatur um 10 K etwa verdoppelt die Rate einer Reaktion, und Erhöhung der Temperatur von 20 K wird die Rate vervierfachen:
(1)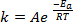
Gleichung (1) findet sich oft in ihrer logarithmischen Form:
(2)
wo k ist die Rate der chemischen Reaktion, A ist der Frequenzfaktor (in Bezug auf die Häufigkeit der molekulare Kollisionen) Eein ist für die Reaktion erforderliche Aktivierungsenergie, R ist die ideale Gaskonstante und T ist die Temperatur, bei der die Reaktion stattfindet.
Eine höhere Temperatur bedeutet daher, dass eine Reaktion viel schneller abgeschlossen ist. Jedoch in einigen Fällen ist es wünschenswert, Reaktionen bei niedrigen Temperaturen, trotz der senkende Wirkung auf die Rate der Reaktion durchzuführen. Einige Szenarien sind in diesem Zusammenhang erläuterten weiter unten.
Wenn es sinnvoll, eine Reaktion unter Raumtemperatur ausgeführt wird, verwenden Chemiker kühlende Bäder um eine bestimmte Temperatur oder Temperaturbereich beizubehalten. Durch die Platzierung der Reaktionskolben innerhalb einer entsprechenden kühlendes Bad sind Reaktionen auf eine gewünschte Temperatur abgekühlt. Die Reagenzien in der Reaktion kommen nie in direktem Kontakt mit den Chemikalien in das kühlende Bad. Das kühlende Bad kann bestehen aus einem kryogenen (Kühlung) Bauteil (z. B. Eis, Trockeneis oder flüssigem Stickstoff) oder möglicherweise eine Mischung der kryogenen Komponente mit einem bestimmten Lösungsmittel und/oder ein Additiv Salz. Das Lösungsmittel soll effektiv Temperatur das Kühlmittel auf der Reaktionskolben zu übertragen, und der Zweck des Zusatzstoffes ist der Gefrierpunkt des Gemisches zu senken (oder drücken). (Beachten Sie, dass es möglich ist für einen Stoff ein Lösungsmittel und Zusatzstoff sein.)
Verfahren
Kühlende Bad Einrichtung
Bereiten Sie für eine allgemeine Konfiguration kühlende Bad Wahl vor, wie unten beschrieben und Tauchen Sie den Reaktionskolben in die Wanne (Abbildung 1). Füllen Sie nicht ganz Bad Behälter, aber lassen Sie genügend Platz zum Eintauchen der Reaktionskolben zu ermöglichen.
Hinweis: Wenn Feuchtigkeit empfindlich reagiert, seien Sie vorsichtig, wenn Sie die Flasche oder einem anderen Teil des Geräts (z. B.
Anwendung und Zusammenfassung
Wann ist es sinnvoll, eine Reaktion bei einer niedrigen Temperatur ausgeführt?
Zur Beantwortung dieser Frage lassen Sie uns untersuchen, vier verschiedene Anwendungen:
Anwendung 1. Manchmal Reaktionen sind auch kräftig und exotherm und das Reaktionsgemisch gekühlt werden muss, um zu verhindern, verschütten und Druck Aufbau durch Gasentwicklung. Eine stark exotherme Reaktion kann auch ein Sicherheitsrisiko werden, da das Reaktionsgemisch schnell...
Referenzen
- Gordon, A. J., Ford, R. A. The Chemist's Companion - A Handbook of Practical Data, Techniques, and References. Chapter 11 (1973) ISBN: 978-0-471-31590-2.
- Rondeau, R. E. Slush baths. J. Chem. Eng. Data. 11, 124 (1966)
pringen zu...
Videos aus dieser Sammlung:

Now Playing
Durchführung von Reaktionen unterhalb der Raumtemperatur
Organic Chemistry
70.6K Ansichten

Einführung in die Katalyse
Organic Chemistry
34.5K Ansichten

Montage eines Reflux-Systems für beheizte chemische Reaktionen
Organic Chemistry
167.5K Ansichten

Übertragen von Lösungmitteln mit der Schlenk-Technik
Organic Chemistry
41.6K Ansichten

Entgasung von Flüssigkeiten mittels Freeze-Pump-Thaw Cycling
Organic Chemistry
56.1K Ansichten

Vorbereiten von wasserfreien Reagenzien und Geräten
Organic Chemistry
79.4K Ansichten

Reinigen von Verbindungen durch Umkristallisation
Organic Chemistry
708.6K Ansichten

Trennen von Gemischen durch Ausfällung
Organic Chemistry
157.8K Ansichten

Fest-Flüssig-Extraktion
Organic Chemistry
237.8K Ansichten

Entfernung von Lösungsmitteln mit dem Rotationsverdampfer
Organic Chemistry
212.9K Ansichten

Fraktionierte Destillation
Organic Chemistry
334.4K Ansichten

Züchten von Kristallen für die Röntgenbeugungsanalyse
Organic Chemistry
32.4K Ansichten

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.7K Ansichten

Säulenchromatographie
Organic Chemistry
360.1K Ansichten

Kernspinresonanzspektroskopie (NMR-Spektroskopie)
Organic Chemistry
247.8K Ansichten
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten
