进行室温下反应
Overview
资料来源: 实验室的博士达纳拉什-的威廉与玛丽学院
通过示范: 马特 · 史密斯
当新债券在化学反应过程中形成的时它需要涉及的物种 (原子或分子) 能在很近的地方,过来撞到另一个。这些物种之间碰撞是更加频繁和有效速度越高这些分子正。一个广泛使用的法则,它扎根在阿伦尼乌斯方程1,国家提高温度 10 K 将大约增加一倍率的一种反应,和提高温度 20 K 将翻两番的速度:
(1)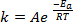
方程 (1) 经常发现在其对数的形式:
(2)
在哪里k是化学反应的速率、 A是频率因子 (有关分子的碰撞频率)、 E是所需的反应的活化能, R为理想气体常数,而T是的温度的反应发生。
因此,较高的温度意味着反应完成得更快。尽管如此,在某些情况下是需要进行反应在低温下,尽管对反应速率的降低影响。几个场景这方面详细阐述了下文进一步。
它有用运行室温下的反应时,化学家使用冷却浴保持一定的温度或温度范围。通过放置烧瓶内适当的冷却浴到所需温度反应冷却。试剂在反应中的不会直接接触冷却浴中的化学物质。冷却浴可能包含单一的低温 (冷却) 组件 (如冰、 干冰或液态氮),或可能具有某些溶剂和/或添加剂盐的低温组件的混合物。溶剂的目的是有效地转移到烧瓶,冷却介质温度和添加剂的目的是降低 (或降低) 冰点的混合物。(请注意,有可能为一种物质是一种溶剂和添加剂)。
Principles
记得溶液在较低的温度比纯液体结冰,这由称为凝固点降低的依数性。凝固点降低影响溶质 (添加剂) 添加到液体的溶剂的量成正比。这种效应,被描述由方程 (3):
(3) ΔDTf = Tf (溶剂) − Tf (解决方案) = Kf × m
ΔDTf是凝固点降低和描述的本身,而与添加剂/溶质溶液冻结的溶剂温度的差异。
Kf是冰点抑郁常数系统,和m是质量,溶液的摩尔浓度。化学家使用对他们有利此效果可以创建的不同温度下的多样性相对容易和成本效益。
通过冷却槽的温度波动。必须监测浴,并作出必要的调整。为了获得最佳结果,应很好绝缘浴容器本身。有空的时候,杜瓦瓶应该用于冷却浴。在杜瓦瓶的缺席,有可能在玻璃或橡胶的容器,容器 (例如使用铝箔或一条毛巾) 尽可能作为最佳绝缘设立浴。使用的容器在所需温度热稳定所需要应该不会开裂。
不同温度低于室温下,在化学实验室设置相对经济和简单的实现存在着许多不同的浴差异。
- 为仅略低于零 (但如果有必要到 55 ° C) 的温度采用不同剂量的盐冰水浴将服务。
- 为-78 ° c 的温度,采用干冰浴在不同溶剂中。
- 可以通过使用液态氮获得温度低于-78 ° C 到-196 ° C。
设置这些冷却浴是相对简单和程序,包括在此文档的末尾。
冰水浴
这种类型是浴的非常容易设置和每个本科教学实验室中可用。有很多的灵活性所浴容器使用,因为冰浴没有达到非常低的温度和开裂一艘船没有风险的类型。
虽然冰水本身的温度为 0 ° C,熔点抑郁症可以通过另外的某些盐如氯化钠、 氯化镁2或氯化2。取得的最终温度会发生变化,并且可以调整量每 100 克的冰所用的添加剂。常见的冰浴是冰的一个与氯化钠作为添加剂 33 g NaCl 的每 100 克的添加位置。通过这种方式取得的最终温度是在-20 ° c。冰水浴能达到的最低温度是冰的在-55 ° C,得到了增加 143 g 的氯化2六水合物每 100 克。
乾冰浴
干冰是固态二氧化碳和 sublimes 在温度-78 ° c。这是一个相当便宜的低温剂、 容易获得的多个实验室。高效传热这温度对反应釜,溶剂是需要具有熔点为-78 ° C 以下。溶剂与更高的熔点或 mp,(更好地称为凝固点在这种情况下) 也可以使用,导致更高的浴温度。
常用的干冰浴中溶剂是丙酮 (mp =-95 ° C),这是现成的和便宜。乾冰浴在丙酮中保持一段时间,其长度取决于绝缘程度-78 ° C 温度。这是最常见的干冰浴系统。
对于较高温度干-冰浴,使用溶剂具有较高的凝固点。获得性的浴温度并不总是等于溶剂的凝固点。请参阅表 2为温度不同系统获得的。
由于达成的这种类型的浴的温度低,低温保护应总是戴手套时处理乾冰。
乾冰浴浴船理想是杜瓦瓶。如果杜瓦瓶不可用,则使用玻璃、 橡胶或不锈钢制成的容器,但要知道不将非常最佳的保温和浴将需要更经常调整。
| 乾冰冷却浴温度 | |
| 混合物 | T (° C) |
| p-二甲苯/干冰 | + 13 |
| 环己烷/乾冰 | + 6 |
| 苯/乾冰 | + 5 |
| 乙二醇/乾冰 | -15 |
| 四氯化碳/乾冰 | -23 |
| 3-庚酮/乾冰 | -38 |
| Acetonenitrile/乾冰 | -42 |
| Cylcohexanone/乾冰 | -46 |
| 二乙基卡必醇/乾冰 | -52 |
| 氯仿/干洗冰 | -61 |
| 卡必醇乙酸酯/乾冰 | -67 |
| 乙醇/乾冰 | -72 |
| 丙酮/乾冰 | -78 |
| 异丙醇/乾冰 | -78 |
表 2。不同的干冰浴混合物的列表。
液态氮浴
当非常低的温度,低于干-冰浴,渴望使用液氮浴。液态氮是熔点-196 ° C,没有额外的溶剂使用时是浴的温度为低温剂。注意,与干冰,N2是一种液体和添加剂溶剂用于传热均匀不是必要的。如果所需温度高于-196 ° C,然后各种不同的有机溶剂用于将导致在不同的温度下,类似与干冰浴的情况一样的混合物。请参阅表 3为温度不同系统获得的。
由于液体 N2浴的温度很低,只有杜瓦瓶应该用作浴船只,总是戴着手套工作,当处理此低温的代理。

表 3。列表的液氮浴用不同溶剂。2
Procedure
冷浴设置
适用于一般的设置,如下所述准备选择冷却浴和烧瓶沉浸在浴 (图 1)。不要所有的方式,填补浴船只,但留有足够空间,以便反应瓶浸泡。
注: 如果反应是水分敏感,要非常小心,当添加试剂瓶或任何其他部分的装置 (例如下降漏斗)。如果玻璃器皿沉浸在冷却浴生成开放,则,然后室温空气迅速内流,并进行中的水分。

图 1。在三颈圆底烧瓶与下降漏斗,温度计在惰性气氛下的冷却浴设置的示例。
1.使冰水浴
- 加冰水冷浴,权衡和选择在浴容器添加适量的冰。杜瓦是有用但不是必要。该船可塑料、 橡胶 (例如一桶),或玻璃 (例如大结晶菜)。对于这个实验,添加到 1 L 容器 500 克冰毒。
- 权衡适量的添加剂咨询冰浴 (表 1),并将这种添加剂添加到冰。对于这个实验,权衡和浴船只的 NaCl 添加 165 g (= 5 × 33 g)。
- 添加少量的去离子水浴容器和彻底使用搅拌棒搅拌。添加只是足够的水,直到所有的冰被覆盖。
- 检查以确保已达到所需的温度的温度计。如有必要,请调整添加剂的量。洗澡不会维护其温度很长时间和需要于频繁的关于每个 20 — — 30 分钟的时间间隔内进行调整。为此,它可能需要移液器关闭一些在浴缸里的水和添加更多的冰和添加剂。
| 物质 | g/100 g H2O | 最终温度 (° C) |
| Na2CO3 | 20 | -2.0 |
| NH43号 | 106 | -4.0 |
| NaC2H3O2 | 85 | -4.7 |
| NH4Cl | 30 | -5.1 |
| 纳米3 | 75 | -5.3 |
| Na2S2O3 ● 5 H2O | 110 | -8.0 |
| CaCl2 ● 6 H2O | 41 | -9.0 |
| 氯化钾 | 30 | -10.9 |
| KI | 140 | -11.7 |
| NH43号 | 60 | -13.6 |
| NH4Cl | 25 | -15.4 |
| NH43号 | 45 | -16.8 |
| NH4SCN | 133 | -18.0 |
| 氯化钠 | 33 | -21.3 |
| CaCl2 ● 6 H2O | 81 | -21.5 |
| H2那么4 (66.2%) | 23 | -25 |
| 溴化 | 66 | -28 |
| H2那么4 (66.2%) | 40 | -30 |
| C2H5OH (4°) | 105 | -30 |
| 氯化镁2 | 85 | -34 |
| H2那么4 (66.2%) | 91 | -37 |
| CaCl2 ● 6 H2O | 123 | -40.3 |
| CaCl2 ● 6 H2O | 143 | -55 |
表 1。盐冰获得通过混合盐和水或冰在特定的温度和指定的数量的冷却混合物。1
2.使乾冰浴
- 穿上低温保护手套和护目镜。总是努力实践这个处理乾冰时,永远不会触摸用赤裸的双手,因为它可以迅速烧伤皮肤,并导致冻伤。
- 为与容量约 1 升的浴船只,采取约 1/3 块的干冰 (通常在 ~ 2 磅块中可用) 和分成几个较小的部分。
- 将块干冰添加到浴船只。
- 慢慢地把有机溶剂 (如丙酮) 干冰边用玻璃棒搅拌。还有由于 CO2气体发展蓬勃嘶嘶声。
- 继续慢慢添加溶剂和翻炒至均匀浆形式和大部分的干冰溶解。这是为了确保传热反应瓶是尽可能均匀。
- 冷温度温度计插入浴以确保达到所需的温度。
- 在固定的时间间隔检查干冰浴并添加更多块的干冰时浴温度升高注意到。间隔时间取决于程度的隔离,但通常是围绕每 45-60 分钟。
3.使液氮浴
- 穿上低温保护手套和护目镜。在处理液态氮,因为它可以迅速燃烧的皮肤组织和眼液体,导致冻伤或永久性眼睛损伤时,总是和这个练习。
- 无添加剂洗个澡,添加适量的 N2对杜瓦瓶获得温度-196 ° c。移动到步骤 3.3 如果这是理想的温度。
- 与添加剂洗个澡,有机溶剂的选择 (参考表 3寻找合适的温度合适的溶剂) 首先向添加杜瓦瓶,然后慢慢地将液体 N2添加到溶剂。
- 冷温度温度计插入浴以确保已达到所需的温度。与其他澡堂,杜瓦瓶内液体的 N2浴一直几个小时的时间保持着它的温度。
- 在适当的间隔 (几个小时),看看是否需要更多 N2检查浴缸的水龙头。
Application and Summary
何时是有用,在低温下运行的反应?
为了回答这个问题让我们调查四个不同的应用程序:
应用程序 1。有时反应太严厉和放热反应,反应混合物必须冷却以防止溢出和压力增大气体发展。高放热反应也能成为安全隐患,如反应混合物可以迅速沸腾 (许多有机溶剂通常有低沸点) 和喷出。这很常见的应用是淬火或工作上步在最初进行反应在无水条件下反应与水和酸在顺序发生作用的最终产品,并关闭任何剩余的活性中间体和反应物反应的末尾。例如,在格氏试剂反应中,一个非常普遍的反应在有机化学中,末尾的淬火步将需要冷却,即使在 0 ° C 冰水浴就够了:
(4)
应用程序 2。冷却可以也需要添加步骤的反应,初时放热反应,否则会在沸腾的有机溶剂。这是不可取的因为反应最好在溶剂中进行。不必添加更多的溶剂,以补偿损失是溶剂的不仅浪费不合算也乏味中许多反应溶剂需要事先干燥步骤,使他们无水。此外,有可能对某些试剂热分解温度的升高。
为了避免这些事件在放热反应,试剂是经常添加滴注射器或下降漏斗到一瓶含有另一剂在溶剂中,同时搅拌和冷却。这种方式,另外可以停止任何时候如果反应变得太剧烈。通常情况下,反应必须低于 0 ° C 冷却和冰水冷浴是不够的。
一个例子为反应,如有必要对二异丙胺形成锂稳定化作用 (LDA) 是另外的强大基地 n-丁基锂 (n-丁基)。
(5)
在没有冷却浴正丁基锂可能分解达到更高的温度:
(6)
应用 3.在一些化学反应中还有多个可能的产品所造成的竞争的化学通路。一种产品可能与一个更稳定的过渡状态,需要较少的激活能量 (Ea1) 是通路的结果,而其他产品可能需要更多的活化能 (Ea2) 但整体更加稳定。前者称为动力学的产品,而后者称为热力学 (TD) 产品 (见图 2中的能量图)。
通过控制反应温度,我们可以控制这些产品中的哪一个形成。因为动力学产品要求少活化能,它是在低温下形成的产物。进行反应在低温常在热力产品确保形成动力学的产品。
经典的例子,在领土的烯醇负离子化学是 2-甲基环己酮与不同基地在不同反应条件下的反应。反应物是不对称的酮,因此具有 α-氢的两种不同类型。小的基地,如氢氧化钠将在更高取代边,结果在更加稳定、 热力学烯 (7) 酮。基地,更含有空间位阻要求,将对阻小方,造成动力学烯醇 (8) 酮。时在-78 ° C 与室温进行反应形成的动力学烯醇会有多更高的产量。然后可以与适当的亲电性,如 methyiodide,反应烯醇的两种形式,形成了如下所示的 α-烷基化的产品。
(7)(8)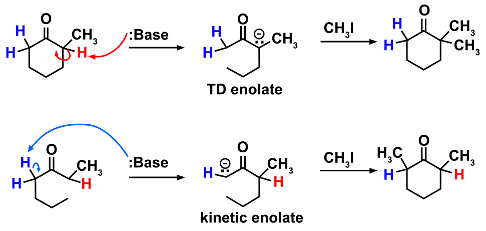
用于获取的动力学烯醇含有空间位阻要求基地经常是的 LDA,编写该早些时候示计划 (5)。它是重要的是温度控制到-78 ° C,以防止回热力学烯醇负离子平衡的动力学烯醇。(注: 那里是没有任何意义-78 ° C,比其他的温度,它很容易获得由干的冰浴中丙酮。)
除了温度控制,另外秩序和试剂的方式是至关重要的。为了获得最佳结果有利于动力学烯醇,酮反应物溶液是滴加入溶剂的 LDA 基地。无水溶剂用于与 LDA 的反应通常是四氢呋喃。示例反应计划 (9) 所示。
(9)
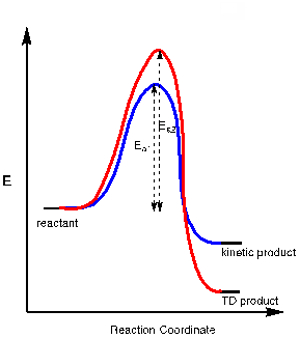
图 2。能量图反应的动力学和热力学的产品。
应用程序 4.在某些情况下是可以调节的温度与试剂反应性。例如考虑酯的减少。用强氢化物还原剂氢化铝锂 (LAH) 反应导致所有的方式到各自的伯醇 (10) 酯的减少。但是,使用笨重的氢化物还原剂丁基氢化 (族) 允许对各自醛酯的选择性还原。可以避免过度减至伯醇,所以只要反应温度保持在低于-78 ° C (但更下降到-90 ° C),只有一个当量相当于族是用 (12)。在-70 ° C 以上的温度下,族变得太被动,并会减少酯对伯醇 (11)。
(10)-(12)
References
- Gordon, A. J., Ford, R. A. The Chemist's Companion - A Handbook of Practical Data, Techniques, and References. Chapter 11 (1973) ISBN: 978-0-471-31590-2.
- Rondeau, R. E. Slush baths. J. Chem. Eng. Data. 11, 124 (1966)
跳至...
此集合中的视频:

Now Playing
进行室温下反应
Organic Chemistry
70.7K Views

催化导论
Organic Chemistry
34.6K Views

程序集的激烈化学反应回流系统
Organic Chemistry
168.3K Views

溅镀转移的溶剂
Organic Chemistry
41.7K Views

随着冻融泵循环脱气液体
Organic Chemistry
56.3K Views

制备无水试剂和设备
Organic Chemistry
79.4K Views

重结晶法净化化合物
Organic Chemistry
710.1K Views

通过沉淀混合物的分离
Organic Chemistry
158.0K Views

固-液萃取
Organic Chemistry
238.1K Views

旋转蒸发来去除溶剂
Organic Chemistry
213.0K Views

分馏
Organic Chemistry
334.8K Views

X 射线衍射分析晶体生长
Organic Chemistry
32.9K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
290.1K Views

柱层析法
Organic Chemistry
360.9K Views

核磁共振 (NMR)
Organic Chemistry
248.9K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。