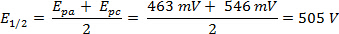サイクリックボルタンメトリー (CV)
概要
ソース: 博士ケーラ緑の研究室-テキサス クリスチャン大学
サイクリック ・ ボルタンメトリー (CV) の実験には、潜在的な電圧を測定しながら現在の範囲のスキャンが含まれます。CV 実験で所定の開始可能性から浸漬、静止電極の電位をスキャンして、最終値 (スイッチングと呼ばれる潜在的な) し次逆スキャンを取得します。これは '繰り返し' 掃引電位を与えるし、電流電位曲線データから派生した対繰返しボルタモ グラムと呼びます。最初の掃引は、'前方スキャン' と呼ばれる、戻る波 '逆スキャン' と呼びます。潜在的な極端は 'スキャン ウィンドウ' と呼ばれます。還元と酸化電流の大きさと電位の形状、濃度、スキャン速度、および実験条件に大きく依存。これらの要因を変化させることによりサイクリックボルタンメトリーはややこしい書き方、電子移動反応の可逆性遷移金属酸化状態の安定性に関する情報と反応性に関する情報を得ることができます。このビデオは、サイクリックボルタンメトリー実験試料の準備も含めて、電気化学セルの設定のための基本的なセットアップについて説明します。単純な循環ボルタンメトリーの実験が表示されます。
手順
1. 電解質溶液の調製
- 0.1 M [Bu4N] から成る電解質貯蔵液 (10 mL) を準備 [BF4] CH3CN で。
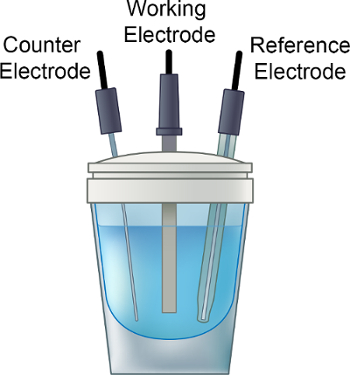
- 電解質溶液を電気化学のバイアルに、小さな攪拌棒を追加し、図 1に示すように、バイアルにキャップを置きます。
- 窒素鉛が電解質溶液であることを確認します。かき混ぜるし、ドガの電解質溶液を酸化還元活性分子酸素を除去する乾燥の N2ガス (~ 10 分) の穏やかな流れに。
- 手順 1.3 の間に注意深く挿入作業電極 (ガラス状炭素など)、カウンター (Pt) および参照電極 (Ag/アグノ3) テフロン セルの上端。携帯スタンドのリード線を適切な電極に接続します。
.css-f1q1l5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:flex-end;-webkit-box-align:flex-end;-ms-flex-align:flex-end;align-items:flex-end;background-image:linear-gradient(180deg, rgba(255, 255, 255, 0) 0%, rgba(255, 255, 255, 0.8) 40%, rgba(255, 255, 255, 1) 100%);width:100%;height:100%;position:absolute;bottom:0px;left:0px;font-size:var(--chakra-fontSizes-lg);color:#676B82;}
結果
参考文献
- Bard, A. J., Faulkner, L. A. Electrochemical methods: Fundamentals and Applications. 2nd ed. New York: Wiley; 833 p. (2001).
- Geiger, W. E., Connelly, N. G. Chemical Redox Agents for Organometallic Chemistry. Chem Rev. 96 (2), 877-910, (1996).
タグ
スキップ先...
このコレクションのビデオ:

Now Playing
サイクリックボルタンメトリー (CV)
Analytical Chemistry
125.2K 閲覧数

試料分析の準備のため
Analytical Chemistry
84.7K 閲覧数

社内基準
Analytical Chemistry
204.8K 閲覧数

標準添加法
Analytical Chemistry
320.1K 閲覧数

検量線
Analytical Chemistry
796.9K 閲覧数

(紫外-可視) 紫外可視分光法
Analytical Chemistry
623.5K 閲覧数

ラマン分光を用いた化学分析
Analytical Chemistry
51.2K 閲覧数

蛍光 x 線 (XRF)
Analytical Chemistry
25.4K 閲覧数

炎イオン化検出ガスクロマトグラフィー (GC)
Analytical Chemistry
282.0K 閲覧数

高速液体クロマトグラフィー (HPLC)
Analytical Chemistry
384.5K 閲覧数

イオン交換クロマトグラフィー
Analytical Chemistry
264.6K 閲覧数

キャピラリー電気泳動 (CE)
Analytical Chemistry
93.9K 閲覧数

質量分析への紹介
Analytical Chemistry
112.3K 閲覧数

走査型電子顕微鏡 (SEM)
Analytical Chemistry
87.2K 閲覧数

ポテンショスタット/Galvanostat を使用して担持触媒の電気化学測定
Analytical Chemistry
51.4K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved